Figure 7.
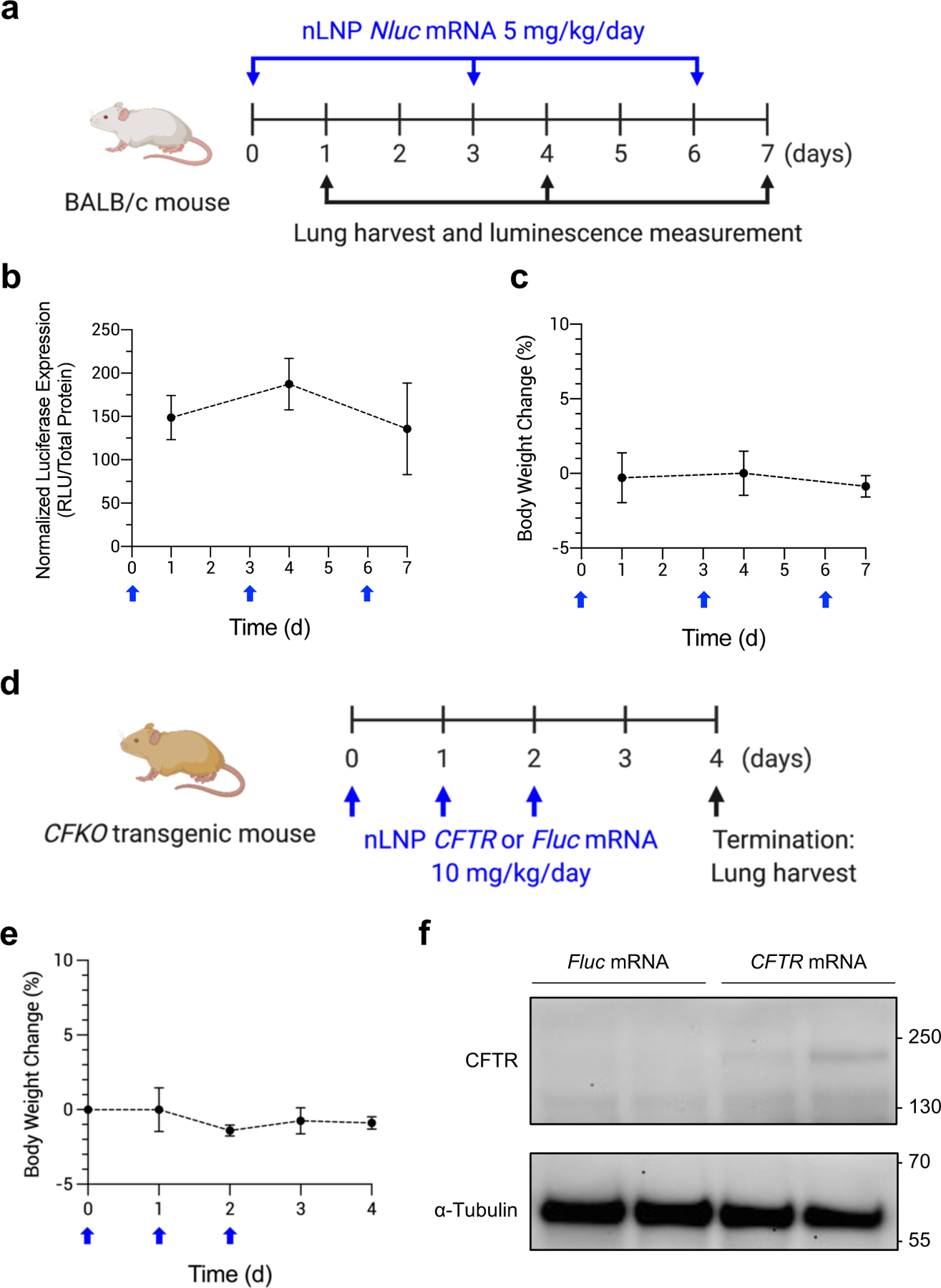
Repeated inhalation of nLNP to sustain production of therapeutic protein. (a) A dosing regimen for persistent inhalation of nLNP encapsulating Nluc mRNA. nLNP was administered via inhalation every 3 days at a dose of 5 mg/kg/day mRNA (blue arrows). At 24 h after each dose, mouse lungs were collected to measure luciferase expression (black arrows). (b) Normalized luciferase expression in mouse lungs and (c) body weight change of BALB/c after repeat dosing (blue arrow). Data were presented in mean ± standard deviation (n=4–12). (d) A dosing regimen for CFTR mRNA delivery via inhalation. nLNP encapsulating CFTR or Fluc mRNA was administered to CFKO mice daily for 3 d (blue arrows), followed by harvesting lungs to detect CFTR proteins (black arrow). (e) Body weight change of CFKO transgenic mice after repeat dosing (blue arrow). (f) Western blot images after immunoprecipitation using an anti-CFTR antibody. mRNA delivered by nLNP is noted above the images. Upper and lower blots were probed using anti-CFTR and anti-α-Tubulin antibodies, respectively. Approximate molecular weights are marked on the left sides of the images.
