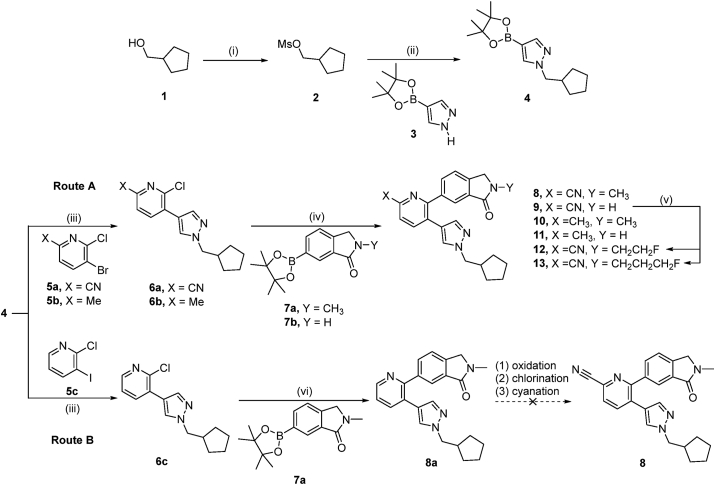
Scheme 1.
Reagents and conditions: (i) 2 equiv. MsCl, 4.4 equiv. Et3N, EtOAc, 0 °C to room temperature, 30 min, 95%; (ii) 1.5 equiv. 3, 1.8 equiv. NaH (60%), DMF, room temperature to 60 °C, 3 h, 75%; (iii) 1.2 equiv. 5a, 5b or 5c, 20% (mol/mol) Pd(dppf)Cl2, 2.5 equiv. K2CO3, 1,4-dioxane/H2O (3/1), room temperature, 10 min, 100 °C, 3 h, 53% for 6a, 65% for 6b, 41% for 6c; (iv) 1.2 equiv. 7a or 7b, 20% (mol/mol) Pd(dtbpf)Cl2, 3 equiv. K2CO3, 1,4-dioxane/H2O (3/1), room temperature, 10 min, 100 °C, 3 h, 64% for 8, 66% for 9, 53% for 10, 26% for 11; (v) 1.7 equiv. NaH, THF, room temperature to 60 °C, 4 h, 2 equiv. ICH2CH2F for 12, 39%, 2 equiv. ICH2CH2CH2F for 13, 37%; (vi) 1.2 equiv. 7a, 20% (mol/mol) Pd(dtbpf)Cl2, 3 equiv. K2CO3, THF/H2O (3/2), room temperature, 10 min, 60 °C, 5 h, 28%. DMF, N,N-dimethylformamide; dppf, 1,1′-bis(diphenylphosphino)ferrocene; dtbpf, 1,1′-bis(di-tert-butylphosphino)ferrocene; THF, tetrahydrofuran.