Figure 4.
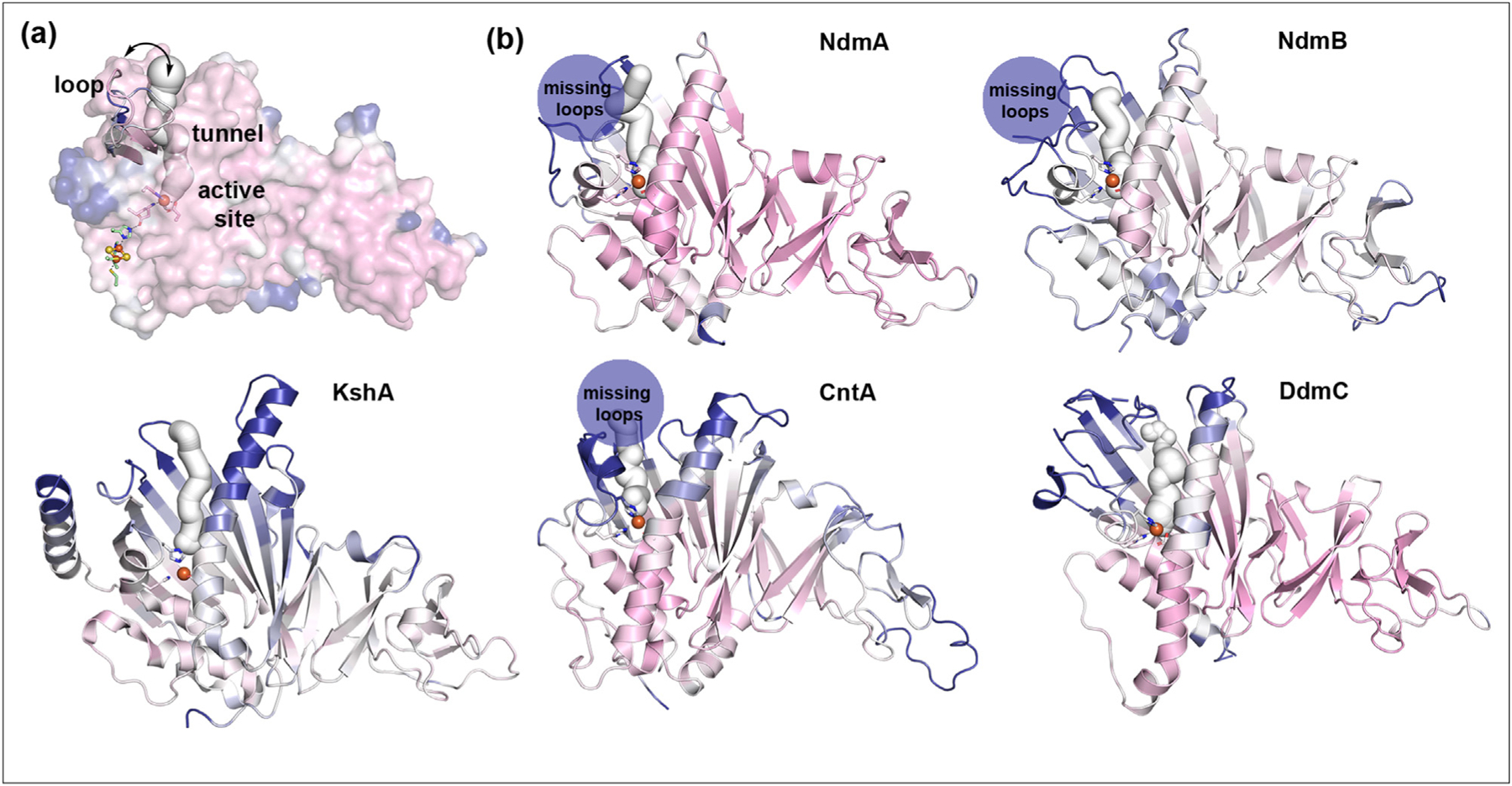
Three architectural regions important to the site-selectivity of the catalyzed reactions of SxtT and GxtA are also found in other Rieske oxygenases. (a) In SxtT and GxtA, residues found in the active site, on the flexible loop that connects β13-to-β14, and lining the tunnel contribute to the site-selectivity of the catalyzed reactions [37,38]. The different loop orientations of SxtA and GxtA are both overlaid with the surface and can also be seen in Figure 3g. (b) In other structurally characterized α3 Rieske oxygenases, a flexible loop, as shown by a plot of the B factors (plotted on a scale of 20–60 Å2 and colored from pink-white-blue), can also be observed in the same region as SxtT and GxtA. In these proteins, tunnels can also be computationally identified [37]. The Rieske oxygenases shown here, in order, are NdmA and NdmB (PDB: 6ICK and 6ICL) [31], KshA (PDB: 4QCK) [34], CntA (PDB: 6Y9D) [39], and DdmC (PDB: 3GKE) [40]. In several cases, the mobility of the loop in the structure resulted in it not being modeled and is indicated by a dark blue circle that says “missing loop”.
