Figure 4. Fasting-induced hyperphagia depends on AgRP circuit integrity.
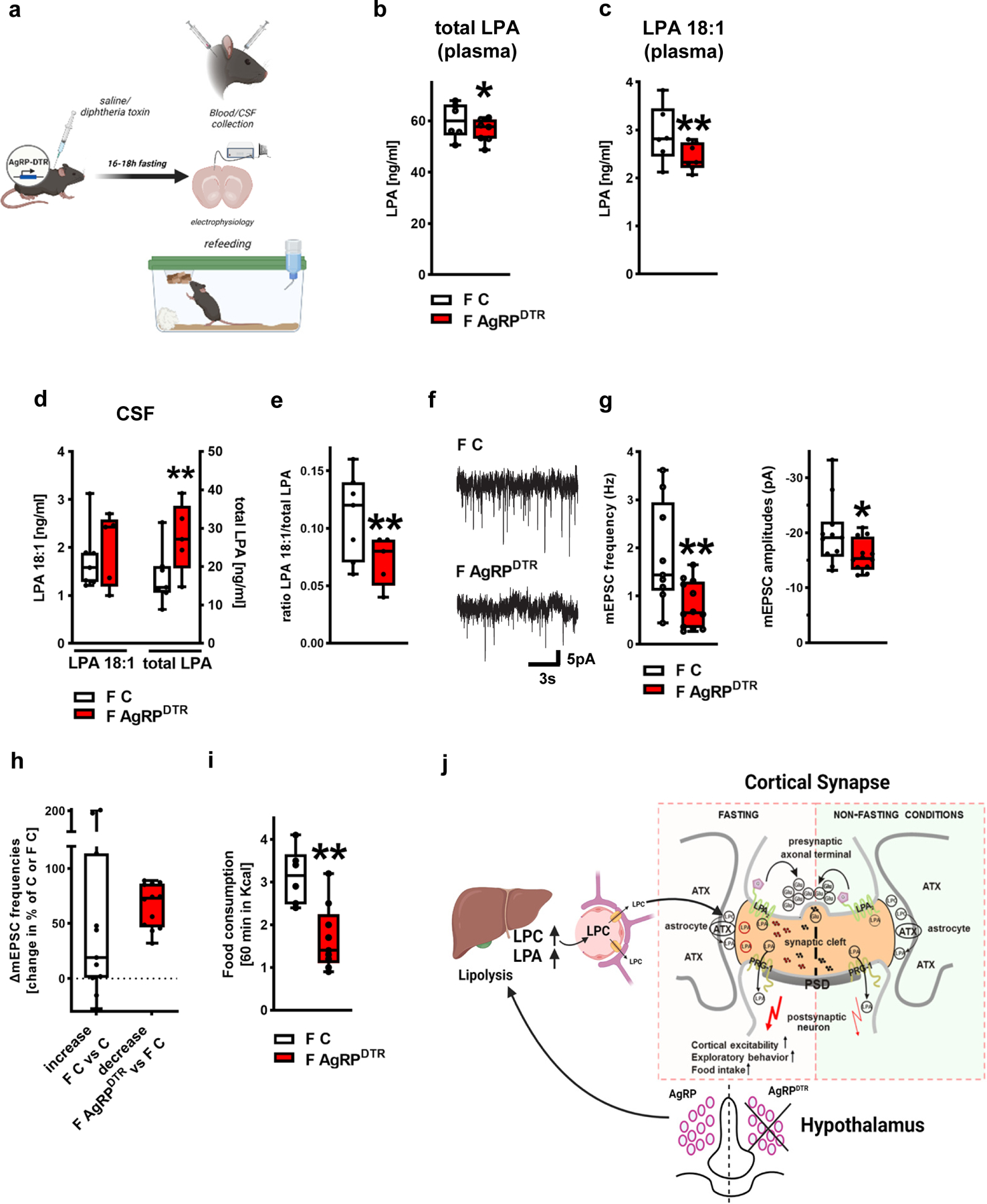
a. Experimental design.
b. Total LPA blood plasma levels were significantly decreased in fasted AgRPDTR (F AgRPDTR) mice (F C, n= 6 fasted controls (F C) and 7 fasted AgRPDTR mice, group differences: 81.0%, Bayesian analysis).
c. Analysis revealed reduced plasma LPA 18:1 levels in fasted AgRPDTR mice (n = 6 F C and 7 F AgRPDTR mice, group differences: 90.5%, Bayesian analysis).
d. CSF LPA 18:1 levels were comparable in fasted AgRPDTR mice and fasted control animals (left, group differences: 68.4%) while total LPA levels were significantly increased in fasted AgRPDTR mice displaying a high variation (n = 7 fasted control mice and 5 fasted AgRPDTR mice, group differences: 92,6%, Bayesian analysis).
e. CSF levels of the synaptic active LPA 18:1 calculated as ratio to total CSF LPA levels in the corresponding animals showed significant reduction in fasted AgRPDTR mice (n = 7 fasted control mice and 5 fasted AgRPDTR mice, group differences: 93.5%, Bayesian analysis).
f. Original traces displaying mEPSCs from prefrontal cortical neurons from fasted control (F C) and fasted, DT-treated AgRPDTR animals (F AgRPDTR).
g. mEPSCs frequency and amplitudes were reduced in neurons of fasted AgRPDTR animals (n = 9 [frequency] and 11 [amplitudes] of fasted controls (F C) and n = 12 for fasted AgRPDTR mice, P = 0.0077 for frequency and P = 0.044 for amplitudes, two sided t-test).
h. mEPSC changes of fasted controls (n = 9 F C increase compared to mean of non-fasted controls shown in Fig. 1h) and fasted AgRPDTR animals (n = 12 F AgRPDTR reduction compared to mean of fasted controls shown in Fig. 4g, two sided Mann Whitney test) did not reveal significant differences.
i. Fasting-induced hyperphagia was significantly decreased in AgRPDTR mice (n = 6 F C and n = 9 F AgRPDTR, P = 0.0022, two tailed t-test).
j. Scheme of fasting-induced increase of cortical excitability by peripheral produced LPA precursors. Following overnight fasting, glycogen stores in the liver are depleted and lipolysis is increased leading to LPC-release in the blood causing increased LPA-levels after overnight fasting via ATX-dependent synthesis in the blood. Our data suggests that this first step in metabolic adaptation to fasting conditions is regulated by AgRP neurons in the hypothalamus. However, following peripheral release upon fasting, LPC is selectively transported across the blood-brain barrier and is metabolized by astrocytic ATX at glutamatergic synapses to generate local LPA which stimulates presynaptic LPA2-receptors leading to fasting-induced increase of glutamatergic transmission and cortical network excitability. In turn, fasting-induced cortical excitability drives fasting-induced hyperphagia as shown by present data.
Box plots and whiskers show data from min to max, line shows median, points represent individual values. *p<0.05, **p<0.01 or group differences of * >80% or **>90% for Bayesian analysis. Illustration was created with BioRender.
