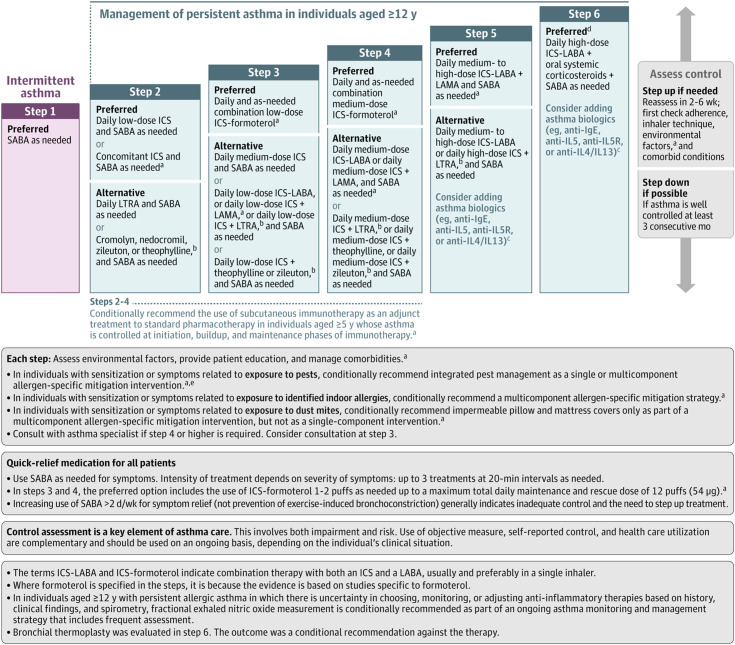
Figure 3.
NAEPP (National Asthma Education and Prevention Program) 2020 recommendations for the management of individuals at least 12 years old with asthma. Note that the NAEPP did not address step one or step six management in their update published in 2020 because of prespecified objectives. aNew recommendation on the basis of the 2020 Asthma Guideline Update. bCromolyn, nedocromil, leukotriene receptor antagonists, inhibitors of 5-lipoxygenase (including zileuton and montelukast), and theophylline were not considered for the update. These have limited availability for use in the United States and/or have an increased risk of adverse consequences and need for monitoring that make their use less desirable. The U.S. Food and Drug Administration issued a boxed warning for montelukast in March 2020 because of adverse effects related to serious behavior- and mood-related changes. cThe AHRQ (Agency for Healthcare Research and Quality) systematic reviews that informed the update did not include studies that examined the role of asthma biologics (anti-IgE, anti–IL-5, anti–IL-5R, and anti–IL-4/IL-13). Thus, this report does not contain specific recommendations for the use of biologics in asthma in steps five and six. dData on the use of long-acting muscarinic antagonist therapy in individuals with severe persistent asthma (step six) were not included in the AHRQ systematic review; thus, no recommendations were made. ePests refers to mice and cockroaches, which were specifically examined in the AHRQ systematic review. Figure reproduced with written permission from Cloutier and colleagues (83). ICS = inhaled corticosteroid; LABA = long-acting β2 agonist; LAMA = long-acting muscarinic antagonist; LTRA = leukotriene receptor antagonist; SABA = short-acting β2 agonist.