Abstract
Rationale
Extrapulmonary manifestations of asthma, including fatty infiltration in tissues, may reflect systemic inflammation and influence lung function and disease severity.
Objectives
To determine if skeletal muscle adiposity predicts lung function trajectory in asthma.
Methods
Adult SARP III (Severe Asthma Research Program III) participants with baseline computed tomography imaging and longitudinal postbronchodilator FEV1% predicted (median follow-up 5 years [1,132 person-years]) were evaluated. The mean of left and right paraspinous muscle density (PSMD) at the 12th thoracic vertebral body was calculated (Hounsfield units [HU]). Lower PSMD reflects higher muscle adiposity. We derived PSMD reference ranges from healthy control subjects without asthma. A linear multivariable mixed-effects model was constructed to evaluate associations of baseline PSMD and lung function trajectory stratified by sex.
Measurements and Main Results
Participants included 219 with asthma (67% women; mean [SD] body mass index, 32.3 [8.8] kg/m2) and 37 control subjects (51% women; mean [SD] body mass index, 26.3 [4.7] kg/m2). Participants with asthma had lower adjusted PSMD than control subjects (42.2 vs. 55.8 HU; P < 0.001). In adjusted models, PSMD predicted lung function trajectory in women with asthma (β = −0.47 Δ slope per 10-HU decrease; P = 0.03) but not men (β = 0.11 Δ slope per 10-HU decrease; P = 0.77). The highest PSMD tertile predicted a 2.9% improvement whereas the lowest tertile predicted a 1.8% decline in FEV1% predicted among women with asthma over 5 years.
Conclusions
Participants with asthma have lower PSMD, reflecting greater muscle fat infiltration. Baseline PSMD predicted lung function decline among women with asthma but not men. These data support an important role of metabolic dysfunction in lung function decline.
Keywords: severe asthma, longitudinal lung function, muscle adiposity
At a Glance Commentary
Scientific Knowledge on the Subject
Body composition analysis from computed tomography (CT) images of the body and chest has been shown to be reflective of outcomes for various diseases and a metric for sarcopenia. What is not well understood is how body composition, as defined from CT, helps predict lung function in individuals with asthma.
What This Study Adds to the Field
We show that CT measurement of the Hounsfield unit density of the erector spinae musculature at the T12 vertebral body level predicts lung function loss over time in women with asthma. This suggests that the metabolome, as quantified by CT body composition, is of potential importance for asthma outcomes.
Lung function decline in asthma is associated with increased morbidity and mortality (1). Previous studies have demonstrated that increased airway inflammation, mucus plugging, frequent exacerbations, smoking history, genetics, and airway remodeling are potential risk factors for lung function decline in asthma (2–7). Recent data from the NHLBI SARP (Severe Asthma Research Program), a longitudinal cohort of subjects with mild to severe asthma enriched for severe asthma (∼60%), showed that failure to improve FEV1% predicted after receiving parenteral corticosteroids was a predictor of severe lung function decline (8). Although several pulmonary predictors of lung function decline in asthma have been investigated, the contribution of extrapulmonary factors has not been well characterized.
Skeletal muscles play a vital role in lung function, and prior studies have demonstrated that sarcopenia is associated with reduced lung function in both normal healthy control subjects and individuals with chronic obstructive pulmonary disease (9, 10). Furthermore, independent of muscle mass, muscle composition or density is an important index of the metabolic quality of the muscle (11). Skeletal muscle adiposity, a metric of skeletal muscle metabolic quality, can be measured noninvasively using noncontrast computed tomography (CT) (12). Lower muscle attenuation on CT is reflective of increased muscle adiposity (12). Skeletal muscle adiposity, as measured using CT imaging increases with age and female sex, is associated with systemic metabolic disturbances such as obesity, insulin resistance, and reduced muscle oxidative capacity, and is an independent predictor of skeletal muscle strength, highlighting the importance of the metabolic quality of skeletal muscle for normal function (11, 13, 14). The paraspinous muscles are a common and validated anatomical region to assess skeletal muscle adiposity on CT (15–17). Previous studies have demonstrated important sex-specific differences in CT attenuation measures of skeletal muscles (14). Given that metabolic dysfunction in asthma is associated with more severe asthma, we hypothesized that participants with asthma have more paraspinous muscle adiposity compared with healthy control subjects and that baseline muscle attenuation on CT would predict longitudinal lung function decline in the SARP cohort (18, 19). We also hypothesized that there would be effect modification of this association by sex.
Methods
Participants with Asthma
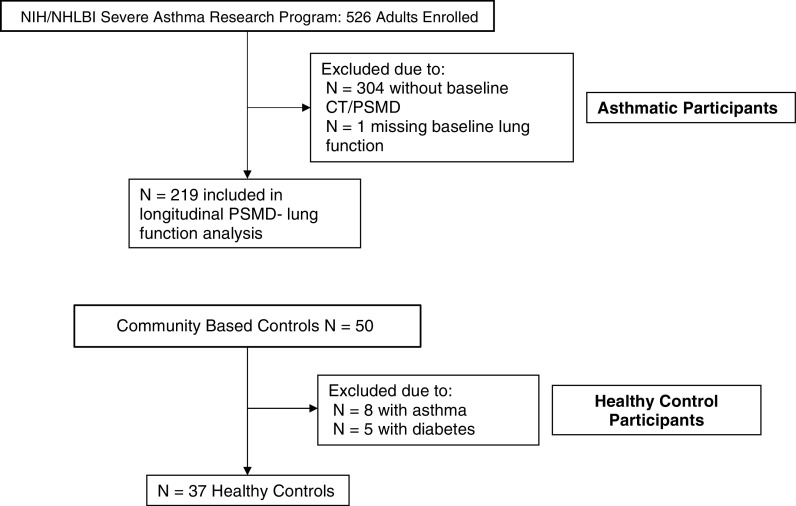
SARP III is a prospective cohort study of 709 participants (526 adults, 183 adolescents) investigating the mechanisms and phenotypes of severe asthma (20). Details of the baseline definitions and characteristics of this cohort have previously been described (21). The subjects were enrolled for their baseline examinations from 2012 to 2015. For this investigation, to avoid the confounding effect of adolescent lung growth, we included only adult (≥18 years of age) participants (8). The study was approved by the institutional review boards of all participating centers. All participants provided written informed consent. Our analysis included the 219 SARP participants who elected to undergo noncontrast chest CT imaging at the baseline visit, with lung function measures and complete baseline covariate data (Figure 1).
Figure 1.
Strengthening the Reporting of Observational Studies in Epidemiology patient flow diagram. CT = computed tomography; PSMD = paraspinous muscle density.
Control Participants
Asymptomatic community participants referred for CT colonography screening were recruited to serve as control subjects for this study (22). Between February 2013 and June 2014, a total of 50 consecutive participants were recruited (22). The study was approved by the institutional review board, and all participants provided oral and written informed consent. Of the 50 consecutive participants, 37 were included in the present study; 8 were excluded for current asthma and 5 for current diabetes mellitus (Figure 1).
Chest CT Methodology
The quantitative CT methods used in this multicenter study have been previously published using the SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) methodology (23). For the control participants, CT data were acquired using a dual-energy 64-slice multidetector row CT scanner (GE Discovery CT750 HD; GE Healthcare). Muscle attenuation was measured in the right and left erector spinae muscles at the 12th rib (paraspinous muscle density [PSMD]) on a picture archiving and communication system workstation (Change; McKesson) using a defined region of interest with an area range of 500–510 mm2 (Figure 2) (24). The average of right and left PSMD was then calculated. Three experienced readers performed all measurements. For reader reproducibility assessment, three blinded readers read 26 scans representing all clinical sites. The intraclass correlation coefficients for intrareader reproducibility were 0.96 for right PSMD and 0.92 for left PSMD. Fleiss’s kappa values for interreader agreement (for all three readers) of dichotomized (above and below the mean) left and right PSMD indicated substantial to almost perfect agreement (0.66 and 0.89, respectively) (25).
Figure 2.
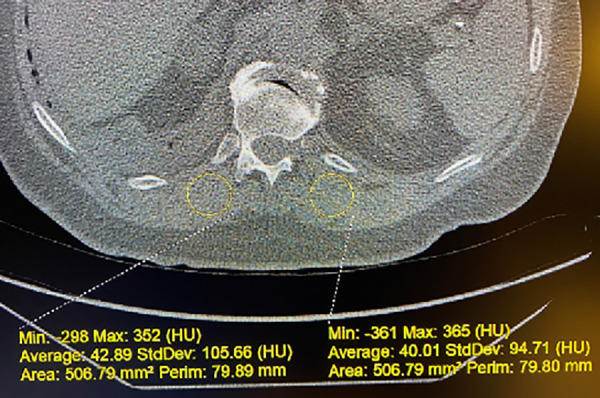
Example of paraspinous muscle density (PSMD) measurement on noncontrast computed tomography images. The method for measurement of the PSMD at the T12 costovertebral junction is illustrated. The right 12th rib’s junction with the T12 vertebral body is the axial location for the measurement on that scan. A 500-mm2 region of interest is used. In this case, right PSMD has an average value of 42.89 HU, and left PSMD has an average value of 40.01 HU. These two measurements are then averaged to yield the mean PSMD for the participant on this specific examination. HU = Hounsfield units; Max = maximum; Min = minimum; Perim = perimeter.
Airway volume measured on CT was calculated using VIDA software (VIDA Diagnostics). Airway generations 3–6 from each participant’s baseline chest CT examination were segmented, and the luminal volumes were calculated. Body surface area (BSA) was calculated using the Du Bois method (26). Airway volume was adjusted for BSA by performing a linear regression of airway volume on BSA and sex (allowing for a different intercept for each sex but similar slopes, as the interaction term was not significant). The residual from the model is used for analyses.
Lung Function Assessment
Details of spirometry and maximum bronchodilator reversibility measures in SARP have been previously described (20). Annual measures were performed according to the American Thoracic Society guidelines. Normative comparisons were assessed using the Global Lung Function Initiative data set (27). Consistent with other reports from SARP, we used postbronchodilator measurements (four puffs of 90 μg/dose of metered-dose inhaler albuterol) as the primary outcome (8).
Biologic Covariates
A complete blood count with differential and eosinophil count was obtained at baseline. Oral corticosteroid use in the preceding year was verified at the baseline examination visit. History of asthma exacerbations was obtained at enrollment on the basis of self-reported increased asthma symptoms that led to the need for systemic corticosteroid courses lasting ≥3 days (28). Whole blood was collected following a 12-hour fast in a subset of participants at examination visit 6 or 7 in Fisher lithium heparin tubes for analysis of plasma glucose and insulin and Fisher plasma ethylenediaminetetraacetic acid tubes for analysis of HbA1c. Plasma IL-6 was measured at baseline in a subset of participants using ultrasensitive ELISA (Quantikine HS Human IL-6 Immunoassay; R&D Systems).
Statistical Analysis
Baseline descriptive statistics are reported as mean (SD) for continuous variables and as percentages for categorical variables. Paired t tests were used to compare baseline continuous variables, the Kruskal-Wallis nonparametric test for ordinal variables, and chi-square tests for categorical variables between the SARP participants in the CT subset and those in the non-CT subset. Comparison of characteristics in participants with asthma and control subjects was done using ANOVA. Associations of factors with PSMD at baseline were analyzed using linear regression. A mixed-effects linear regression model was used to investigate the association of baseline PSMD and lung function trajectory over all annual study visits, adjusting for biologic confounders. This model was fit with the FEV1% predicted measurement at each time and random slopes and intercepts for each participant. A series of models were created by adding potential known biological confounders into each model: model 1 was unadjusted; model 2 was adjusted for age and sex; model 3 was also adjusted for baseline blood eosinophils and asthma exacerbations; model 4 was also adjusted for baseline oral corticosteroid use; and model 5 was also adjusted for baseline body mass index (BMI). All models use the baseline covariate with an interaction term for visit (to assess the effect of the covariate on the slope). These models allow subjects with only baseline data to still contribute to the analyses. The models allow for separate associations for each covariate with the intercept and the slope (interaction of covariate with the time measure). Our lung trajectory primary model results focus on the slope terms, where β values represent the shift in slope per decrease of 10 Hounsfield units (HU) in PSMD. The models are centered on PSMD at 50 HU. The slope term in the model estimates the slope at this PSMD level, and the interaction term represents the shift in this slope as PSMD decreases.
Potential differences in the associations by sex were evaluated by testing for an interaction among sex, visit, and PSMD. The interaction P value was less than 0.10 (Pinteraction = 0.07), which is suggestive evidence of effect modification to warrant stratified models (29). Consistent with reporting guidelines, our results are presented as sex stratified (14, 29, 30). Eosinophil count was log transformed for analyses. IL-6 was measured in a subset of SARP participants at the baseline examination. Serum markers of insulin sensitivity were not measured at baseline but were measured in a subset of participants at examination 6 or 7. Sensitivity analyses were performed on the subsets with IL-6 measured at baseline (n = 151) and markers of insulin sensitivity including glucose (n = 151), insulin (n = 151), homeostasis model assessment–estimated insulin resistance (n = 151), and Hb A1c (n = 169) at examination visits 6 and 7 (see the online supplement). Current or significant smoking history (>5 pack-years if <30 years of age and >10 pack-years if >30 years of age) was an exclusion criterion for the SARP III cohort and therefore was not included in our primary models (20). A sensitivity analysis was performed adjusting for smoking status (ever/never) and duration (see the online supplement). Statistical analysis and figures were generated using SAS 9.4 and SAS/JMP software (SAS Institute). All statistical tests were two-sided, with P < 0.05 indicating statistical significance without adjustment for multiple comparisons.
Results
Participant Characteristics
The characteristics of the 219 participants with asthma who underwent CT imaging (CT subgroup) are shown in Table 1. The subgroup was very similar to the entire SARP III cohort, although the CT subgroup was slightly more likely to have severe asthma (Table 1). These 219 participants contribute 1,132 person-years, with a median follow-up duration of 5 years. The female participants with asthma, compared with the male participants, had higher baseline BMI (33.7 vs. 31.0 kg/m2; P = 0.03), higher prebronchodilator FEV1% predicted (75.9% vs. 65.6%; P < 0.001), and lower (worse) PSMD (41.5 vs. 44.2 HU; P = 0.04) (Table 1).
Table 1.
Baseline Characteristics, Sex Stratified: Entire Severe Asthma Research Program Cohort Compared with Computed Tomography Subgroup
| Full SARP Cohort and CT Subgroup |
Sex Stratified in CT Subgroup |
|||||
|---|---|---|---|---|---|---|
| Full SARP Data | CT Subgroup | P Value | Men | Women | P Value | |
| Analytic sample size, n | 526 | 219 | 73 | 146 | ||
| Age, yr* | 47.6 ± 13.8 | 47.2 ± 14.0 | 0.58 | 46.6 ± 14.5 | 47.5 ± 13.7 | 0.66 |
| Sex† | ||||||
| Male | 174 (33.1) | 73 (33.3) | 0.92 | |||
| Female | 352 (66.9) | 146 (66.7) | ||||
| Duration of asthma, yr* | 27.9 ± 15.7 | 28.0 ± 14.8 | 0.95 | 29.6 ± 16.2 | 27.2 ± 14.1 | 0.25 |
| Primary race† | ||||||
| White | 341 (64.8) | 144 (65.8) | 0.70 | 51 (69.9) | 93 (63.7) | 0.66 |
| Black | 141 (26.8) | 55 (25.1) | 16 (21.9) | 39 (26.7) | ||
| Other | 44 (8.4) | 20 (9.1) | 6 (8.2) | 14 (9.6) | ||
| Body mass index, kg/m2* | 32.5 ± 8.4 | 32.8 ± 8.8 | 0.43 | 31.0 ± 6.7 | 33.7 ± 9.6 | 0.03 |
| Severe asthma† | ||||||
| No | 216 (41.1) | 71 (32.4) | <0.001 | 20 (27.4) | 51 (34.9) | 0.26 |
| Yes | 310 (58.9) | 148 (67.6) | 53 (72.6) | 95 (65.1) | ||
| Maintenance OCS† | ||||||
| No | 456 (86.7) | 185 (84.5) | 0.21 | 61 (83.6) | 124 (84.9) | 0.79 |
| Yes | 70 (13.3) | 34 (15.5) | 12 (16.4) | 22 (15.1) | ||
| Number of controller therapies‡ | 2 (2–3) | 2 (2–3) | 0.06 | 2 (2–3) | 3 (2–3) | 0.53 |
| Exacerbations in prior 6 mo‡ | 1 (0–2) | 1 (0–3) | 0.42 | 0 (0–2) | 1 (0–3) | 0.12 |
| ACT score* | 16.9 ± 4.8 | 16.4 ± 5.0 | 0.07 | 16.2 ± 5.1 | 16.6 ± 5.0 | 0.58 |
| MARS score* | 22.0 ± 3.2 | 22.1 ± 3.1 | 0.69 | 22.2 ± 3.5 | 22.0 ± 2.9 | 0.67 |
| Pre-BD FEV1% predicted* | 72.3 ± 20.4 | 72.5 ± 20.0 | 0.85 | 65.6 ± 18.8 | 75.9 ± 19.8 | <0.001 |
| Maximum albuterol reversibility* | 11.3 ± 7.9 | 11.0 ± 7.8 | 0.39 | 11.4 ± 9.8 | 10.8 ± 6.6 | 0.57 |
| PSMD, HU* | 42.4 ± 9.1 | 44.2 ± 8.5 | 41.5 ± 9.3 | 0.04 | ||
Definition of abbreviations: ACT = Asthma Control Test; BD = bronchodilator; CT = computed tomography; HU = Hounsfield units; MARS = Medication Adherence Report Scale; OCS = oral corticosteroids; PSMD = paraspinous muscle density; SARP = Severe Asthma Research Program.
Distribution for continuous measures is mean ± SD.
Distribution for categorical measures is n (%).
Distribution for ordinal measures is median (interquartile range).
The 37 healthy control subjects had a mean (SD) age of 55.6 (5.0) years and a mean (SD) BMI of 26.3 (4.7) kg/m2. Compared with control subjects, and regardless of sex, participants with asthma were younger (P < 0.001) and had higher BMI (P < 0.001). Fifty-one percent of control subjects were female, and there were no sex differences in age (P = 0.67) and BMI (P = 0.73).
PSMD in Participants with Asthma and Control Participants
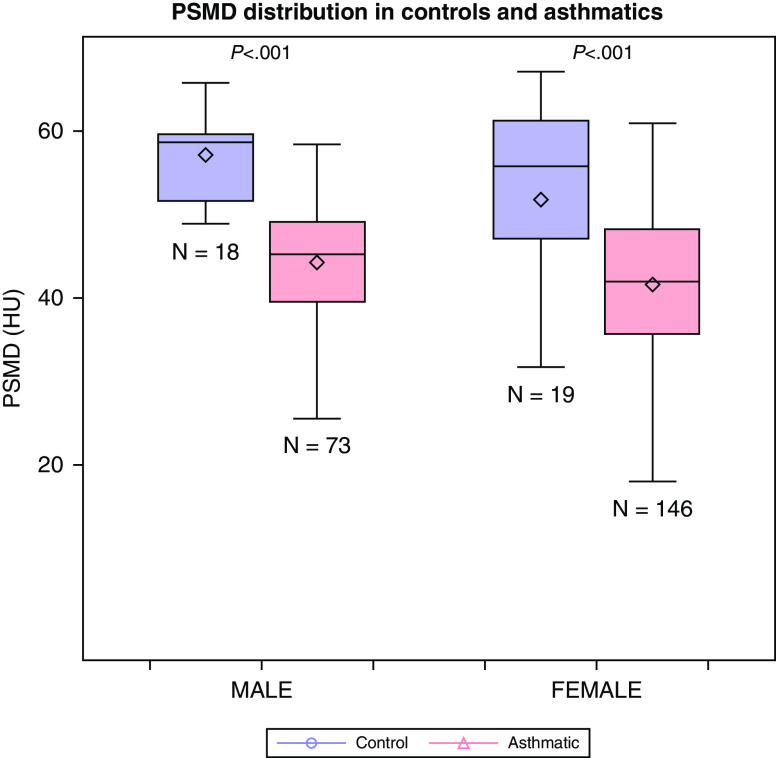
Among control subjects, women had lower PSMD compared with men (mean [SD], 51.8 [14.9] vs. 57.2 [5.0] HU; P = 0.076), with similar findings among participants with asthma: women had significantly lower PSMD compared with men (mean [SD], 41.5 [9.3] vs. 44.2 [8.5] HU; P = 0.04) (Figure 3). Overall, participants with asthma had lower unadjusted PSMD compared with control subjects (mean [SD], 42.4 [9.1] vs. 54.4 [11.4] HU; P < 0.001), this was consistent for both sexes (P < 0.001 for both). In age- and BMI-adjusted models, both female and male participants with asthma had lower PSMD compared with control subjects (P < 0.001 for both).
Figure 3.
Comparison of mean PSMD in control subjects and participants with asthma, stratified by sex. HU = Hounsfield units; PSMD = paraspinous muscle density.
Asthma Characteristics by PSMD Tertile
The descriptive statistics of asthma-related characteristics by baseline PSMD tertile are shown in Table 2. Overall, many of the asthma-related characteristics within each PSMD tertile were similar between men and women, except for age and BMI. The participants in the lowest (worst) tertile of PSMD were older (men: first tertile PSMD [highest] mean [SD] age, 42.0 [13.7] yr vs. third tertile [lowest] PSMD mean [SD] age, 54.3 [10.2] yr [P = 0.005]; women: first tertile PSMD [highest] mean [SD] age, 41.0 [12.3] yr vs. third tertile [lowest] PSMD mean [SD] age, 52.8 [12.9] yr [P < 0.001]) and had higher BMI (men: first tertile PSMD [highest] mean [SD], 27.6 [4.8] kg/m2 vs. third tertile [lowest] PSMD mean [SD], 36.1 [6.9] kg/m2 [P < 0.001]; women: first tertile PSMD [highest] mean [SD], 29.9 [7.8] kg/m2 vs. third tertile [lowest] PSMD mean [SD], 39.0 [10.3] kg/m2 [P < 0.001]). Similarly, participants in the lowest (worst) tertile of PSMD, both men and women, had larger BSAs. Asthma control–related characteristics (exacerbations, Asthma Control Test score) and length of time with asthma did not differ by tertile of baseline PSMD (P > 0.05). BSA-adjusted airway volume was lower in those in the lowest tertile of PSMD (P = 0.03 in men, P = 0.002 in women). Both men and women have airway volumes below expected for their BSA when PSMD is in the lowest (third) tertile and above what is expected when the PSMD is in the highest (first) tertile (Table 2).
Table 2.
Baseline Characteristics of Subjects with Asthma by Tertile of Paraspinous Muscle Density
| Male |
Female |
|||||||
|---|---|---|---|---|---|---|---|---|
| First Tertile (>48 HU) |
Second Tertile (42–48 HU) | Third Tertile (<42 HU) | First Tertile (>46.4 HU) |
Second Tertile (38.2–46.4 HU) | Third Tertile (<38.2 HU) | |||
| Characteristic | (n = 24) | (n = 25) | (n = 24) | P Value | (n = 48) | (n = 49) | (n = 49) | P Value |
| Age at baseline, yr* | 42.0 ± 13.7 | 43.6 ± 16.1 | 54.3 ± 10.2 | 0.005 | 41.0 ± 12.3 | 48.5 ± 13.5 | 52.8 ± 12.9 | <0.001 |
| Body mass index, kg/m2* | 27.6 ± 4.8 | 29.4 ± 5.2 | 36.1 ± 6.9 | <0.001 | 29.9 ± 7.8 | 32.2 ± 8.0 | 39.0 ± 10.3 | <0.001 |
| Body surface area, m2* | 2.04 ± 0.18 | 2.07 ± 0.23 | 2.26 ± 0.25 | 0.002 | 1.86 ± 0.23 | 1.90 ± 0.24 | 2.02 ± 0.26 | 0.005 |
| Asthma duration, yr* | 29.6 ± 17.0 | 29.9 ± 13.7 | 29.3 ± 18.4 | 0.99 | 23.7 ± 12.9 | 28.1 ± 13.7 | 29.6 ± 15.2 | 0.10 |
| Baseline exacerbations in prior 6 mo† | 0 (0–2) | 1 (0–3) | 0 (0–1) | 0.66 | 1 (0–3) | 1 (0–3) | 1 (0–3) | 0.79 |
| Severe asthma‡ | 13 (54.2) | 20 (80.0) | 20 (83.3) | 0.05 | 27 (56.3) | 33 (67.3) | 35 (71.4) | 0.27 |
| Number of controller medications† | 2 (1–3) | 2 (2–3) | 3 (2–4) | 0.03 | 3 (2–3) | 2 (2–3) | 3 (2–3) | 0.66 |
| Maintenance oral corticosteroid use‡ | 1 (4.2) | 4 (16.0) | 7 (29.2) | 0.07 | 5 (10.4) | 10 (20.4) | 7 (14.3) | 0.38 |
| ACT score* | 17.0 ± 5.4 | 16.6 ± 4.2 | 14.9 ± 5.6 | 0.31 | 17.0 ± 4.8 | 17.2 ± 4.9 | 15.5 ± 5.3 | 0.21 |
| Pre-BD FEV1% predicted* | 66.7 ± 21.9 | 66.2 ± 18.4 | 63.7 ± 16.5 | 0.84 | 80.0 ± 20.0 | 77.119.9 | 70.9 ± 18.8 | 0.07 |
| CT airway volume, mm3* | 11,666 ± 2,867 | 10,521 ± 2,767 | 9,737 ± 2,560 | 0.06 | 9,000 ± 3,096 | 9,085 ± 2,292 | 7,452 ± 2,652 | 0.004 |
| CT airway volume, BSA adjusted, mm3*§ | 1,087 ± 2,836 | −80 ± 2,682 | −1,003 ± 2,542 | 0.03 | 540 ± 3,092 | 595 ± 2,277 | −1,124 ± 2,637 | 0.002 |
Definition of abbreviations: ACT = Asthma Control Test; BD = bronchodilator; BSA = body surface area; CT = computed tomography; HU = Hounsfield units; PSMD = paraspinous muscle density.
Distribution for continuous measures is mean ± SD.
Distribution for ordinal measures is median (interquartile range).
Distribution for categorical measures is n (%)
BSA-adjusted airway volume represents the value above or below the expected airway volume for BSA (residual from a linear model).
PSMD and Longitudinal Lung Function Trajectory
To evaluate for the association of PSMD and lung function change, a series of a priori mixed models adjusting for biologic confounders were constructed; β values represent the shift in FEV1% predicted slope per 10-HU decrease in PSMD. In unadjusted models, PSMD was not associated with FEV1% predicted trajectory in men (unadjusted shift in slope: β = 0.01 per 10-HU decrease; P = 0.98) but was associated in women (unadjusted shift in slope: β = −0.59 per 10-HU decrease; P < 0.001). This association remained in a series of models adjusting for biologic confounders (Table 3). In fully adjusted, sex-stratified models, baseline PSMD strongly predicted future lung function trajectory in women (shift in slope per 10-HU decrease: β = −0.47; 95% confidence interval, −0.90 to −0.04; P = 0.03) but not in men (shift in slope per 10-HU decrease: β = 0.11; 95% confidence interval, −0.66 to 0.89; P = 0.77). Expected FEV1% predicted slopes by baseline PSMD tertiles are displayed in Figure 4. In fully adjusted models, for women, if baseline PSMD is in the highest tertile, the expected FEV1% predicted would increase by about 2.9% from baseline through 5 years. However, as PSMD decreases, the slope decreases, eventually showing an FEV1% predicted decline of about 1.8% over the 5 years when baseline PSMD is in the lowest tertile for women. A sensitivity analysis found no attenuation of the association of PSMD and lung function trajectory in women when model 5 included or excluded the respective biomarker in the subsets of participants with available data (see Table E1 in the online supplement).
Table 3.
Association of Baseline Paraspinous Muscle Density and Lung Function Trajectory in the Severe Asthma Research Program
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| β | 95% CI | P Value | β | 95% CI | P Value | |
| Model 1 | 0.01 | −0.59 to 0.61 | 0.98 | −0.59 | −0.94 to −0.25 | <0.001 |
| Model 2 | −0.01 | −0.68 to 0.66 | 0.97 | −0.54 | −0.90 to −0.17 | 0.004 |
| Model 3 | −0.03 | −0.73 to 0.66 | 0.92 | −0.58 | −0.95 to −0.22 | 0.002 |
| Model 4 | −0.05 | −0.79 to 0.68 | 0.89 | −0.58 | −0.95 to −0.22 | 0.002 |
| Model 5 | 0.11 | −0.66 to 0.89 | 0.77 | −0.47 | −0.90 to −0.04 | 0.03 |
Definition of abbreviation: CI = confidence interval.
Model 1 is unadjusted. Model 2 is adjusted for age and sex. Model 3 includes model 2 parameters plus baseline blood eosinophils and asthma exacerbations. Model 4 includes model 3 parameters plus baseline oral corticosteroid use. Model 5 includes model 4 parameters plus baseline body mass index.
Figure 4.
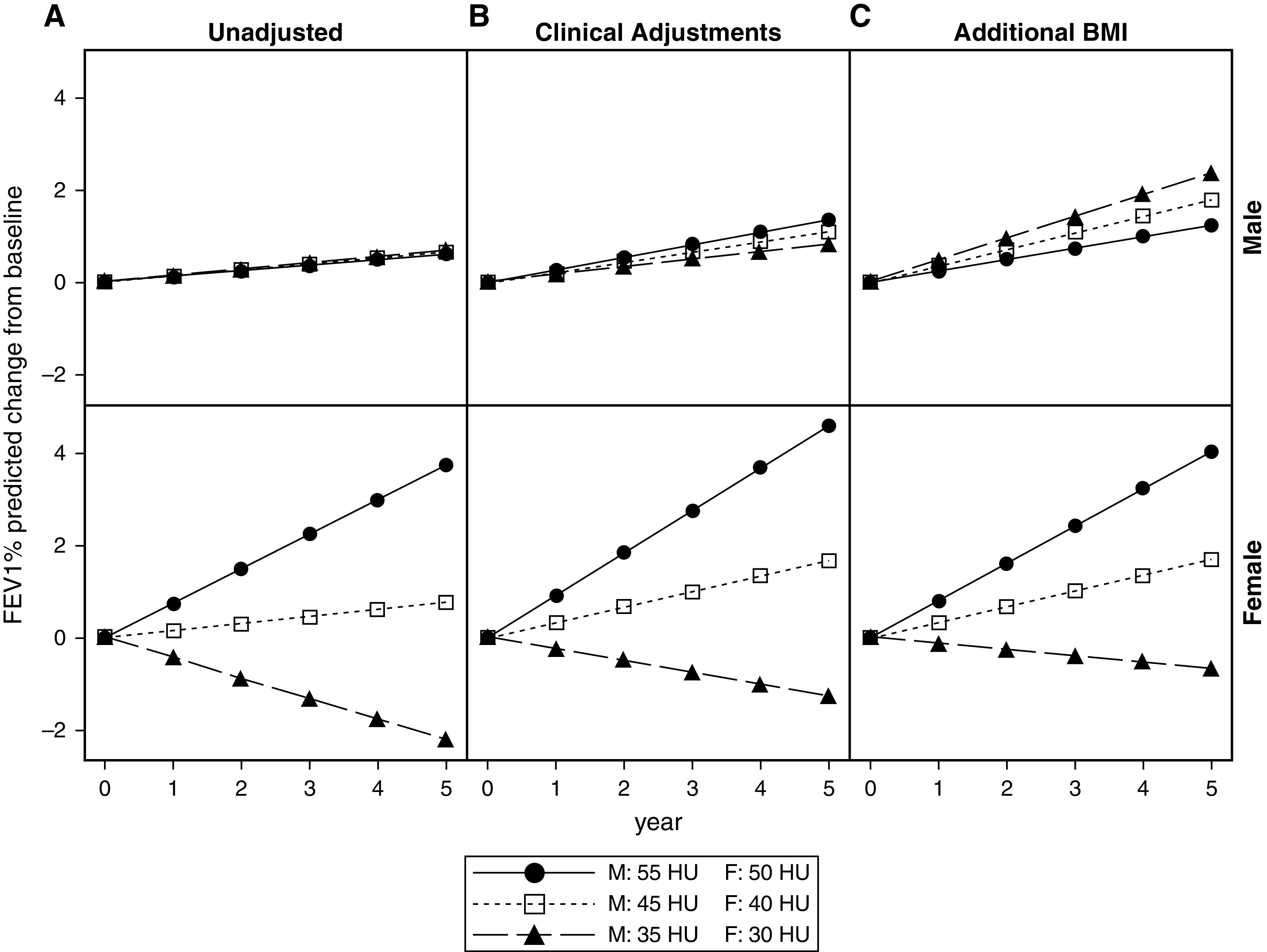
Sex-stratified baseline paraspinous muscle density (PSMD) and lung function decline by tertile of PSMD. The sex-stratified model of tertiles of baseline mean PSMD predicting longitudinal FEV1% predicted slope from mixed-effects models is illustrated. (A) Unadjusted (model 1). (B) Adjusted for asthma clinical variables (age, eosinophils, oral corticosteroid use, and exacerbations; model 4). (C) Also adjusted for BMI (model 5). This shows the trend for change in FEV1% predicted in the SARP (Severe Asthma Research Program) III cohort over the 5-year period of observation according to baseline computed tomography PSMD. Only women showed a significant loss in lung function with lower PSMD. BMI = body mass index; HU = Hounsfield units.
Discussion
In a large, longitudinal, severe asthma cohort with a median 5 years of follow-up, PSMD, an easily measurable, highly reproducible CT imaging biomarker of skeletal muscle adiposity, was lower (higher adiposity) in participants with asthma compared with healthy control subjects and worse in female participants with asthma compared with male participants with asthma. In female participants with asthma, PSMD independently predicted longitudinal lung function decline. Women with asthma had higher baseline BMI compared with men with asthma, and the association of PSMD and lung function decline in female participants with asthma persisted after adjustment for BMI. PSMD and BMI are highly correlated measures, and both reflect systemic metabolic disturbance. Although BMI is a general measure of metabolic disturbance, PSMD represents a tissue-level proxy for metabolic disturbance, and among women with asthma, this measure provides additional predictive capability in lung function trajectory. Adjustment for plasma insulin sensitivity markers and IL-6, an adipokine and a marker of systemic inflammation, in sensitivity analyses did not attenuate the association of baseline PSMD and lung function trajectory in women, suggesting that PSMD may provide additional predictive capability beyond the serum assessments of metabolic disturbance and inflammation.
PSMD offers a reproducible measure that is obtainable on noncontrast CT of the chest, which may have already been obtained, especially in difficult-to-control or severe asthma to identify associated conditions or exclude confounding conditions. Clinically, identification of individuals with asthma at high risk of the morbidity of future longitudinal lung function decline has the potential to influence important clinical management considerations. First, there is heterogeneity in how individuals with asthma perceive asthma control, often having a higher (more uncontrolled) personalized threshold for the definition of control compared with standardized asthma questionnaire definitions (31). Knowledge of higher risks of lung function decline may compel clinicians and patients to increase efforts to identify symptoms in “nonperceivers” to improve congruency with objective measures of asthma control. Second, poor asthma medication adherence and persistence remains a significant clinical problem and is a risk factor for lung function decline (32). Identification of patients at high risk for lung function decline may be informative to both patients and clinicians in the framework of a shared decision-making process in exploring treatment expectations and goals. Third, PSMD identified subjects with asthma at high risk for lung function decline independent of typical airway markers of risks of lung function decline (exacerbation history, blood eosinophils), so early identification of individuals with asthma at higher risk for lung function decline provides an opportunity to target, optimize, and personalize therapeutic interventions to slow lung function decline. Finally, PSMD is a marker of systemic metabolic health. Recent data have demonstrated associations between treatment of metabolic dysfunction in the setting of asthma and improved asthma control. Addressing the metabolic disturbance present in asthma has the opportunity to improve systemic health and asthma control (33).
The association of lung function decline and PSMD may have several potential mechanistic underpinnings. PSMD reflects an imaging-based, tissue-level proxy for the presence of systemic metabolic disturbance. Muscle is the largest source of insulin sensitive tissue in the body and is the primary depot for insulin-mediated glucose uptake (34, 35). Intramuscular fascicle and perimuscular fascicle fat accumulation measured by muscle attenuation on CT imaging are strongly associated with insulin resistance (11). In obese individuals, decreased CT attenuation of skeletal muscle was a more informative imaging marker of insulin resistance compared with abdominal and visceral adiposity imaging measures (11). The metabolic activity of skeletal muscle is heterogeneous, but PSMD is an ideal barometer of metabolic disturbance, as this muscle group is composed primarily of slow-twitch (type I) muscle fibers, which have higher insulin sensitivity than fast-twitch (type II) fibers (36, 37). Although intramuscular fat deposition is a marker of systemic insulin resistance, the fat deposited is metabolically active, through paracrine signaling of TNF-α (tumor necrosis factor-α), creating a vicious circle of intramuscular adipose tissue inducing more insulin resistance in the muscle, leading to further intramuscular adipose accumulation (38). Metabolic disturbance is an emerging risk factor in asthma that is associated with asthma severity and lower lung function (39). In the ARIC (Atherosclerosis Risk in Communities) study, participants with more severe diabetes mellitus regardless of asthma status had lower baseline lung function parameters and more rapid lung function decline over the observational period, implicating a role of metabolic disturbance alone in lung function metrics (40). Mechanistically, the link between systemic metabolic dysfunction and lung function decline has not been fully elucidated. Proposed mechanistic underpinnings include dysanaptic airway growth, which has previously been characterized in obese children with and without asthma; systemic inflammation affecting airway function; and potential deleterious effects of insulin resistance on airway smooth muscle contractility and remodeling (19, 39, 41–43). We observed significantly lower CT-calculated airway volumes of the third- to sixth-generation bronchi in both sexes in the lowest (worst) PSMD tertile, after adjusting for BSA. This may reflect the impact of body habitus on airway patency, the end-organ effects of metabolic syndrome on airway remodeling, or airway tone in participants with asthma with low PSMD. Further research into these mechanisms will be needed. Regardless of the mechanism, PSMD may serve as an early marker of overall systemic metabolic health.
Second, PSMD may represent a marker of overall skeletal muscle quality throughout the body. In the Health ABC (Health, Aging and Body Composition) longitudinal study, lower CT attenuation was associated with reduced skeletal muscle strength, supporting the hypothesis that skeletal muscle adipose infiltration is associated with systemic functional consequences (14). An intensive strength training program increased (improved) muscle attenuation measured on CT and decreased intramuscular adipose tissue in a study of elderly women, supporting the important role of strength training in modifying intramuscular adiposity (44).
Third, inflammatory cytokines (IL-6, TNF-α) are also associated with lower skeletal muscle strength (45). It has previously been shown that individuals with asthma have higher concentrations of these proinflammatory cytokines in the serum compared with those without asthma (46). IL-6 and TNF-α not only drive declines in muscle strength and quality but have direct effects on insulin sensitivity and are hypothesized to serve as a connection in the observation of increased intramuscular adiposity and insulin resistance (47). Taken together, PSMD may be an imaging biomarker reflecting the tissue consequences of chronic inflammation leading to systemic metabolic disturbance with deleterious functional effects on skeletal muscle with sex-specific differences. The strong association of baseline PSMD and future lung function trajectory among female but not male participants with asthma highlights important sex-specific differences of the asthma syndrome and requires future mechanistic studies to further elucidate.
Strengths and Limitations
The present study includes a subset of SARP participants who elected to undergo CT scanning. Analysis of baseline patient characteristics found that participants in the CT subset and non-CT subset were similar, excepting a higher proportion of participants with severe asthma in the CT subset (Table 1). The longitudinal, observational nature of SARP provides a few considerations. The repeated-measures design provides the potential for unbalanced (mistimed) outcome assessments and missing covariate data. However, we used a mixed-effects model approach to allow improved statistical efficiency given the subject-specific random-effects structure and maximum use of the available data, even in those with missing data. This is an observational study; therefore, the described associations do not confirm causation. The mixed-effects regression models were adjusted for measured known confounders; however, unmeasured confounding may result in residual confounding. The primary hypothesis of this study was to investigate whether an extrapulmonary imaging biomarker of skeletal muscle adiposity predicted lung function decline. Given the design of SARP and the baseline PSMD measures, this study cannot delineate the mechanism or exclude a bidirectional relationship in the association of PSMD and lung function decline. The analyses identified some relationships with airway volume, sex, and BMI that could not be fully evaluated with only baseline measures of PSMD. A sensitivity analysis was performed to investigate whether plasma markers of insulin sensitivity or IL-6 would attenuate this association, and we did not find evidence of attenuation. This sensitivity analysis should be interpreted with caution, as the subset of participants with baseline IL-6 was small, which may introduce selection bias. The measures of insulin sensitivity were not collected at baseline but at examination 6 or 7 and thus may or may not be representative of insulin sensitivity measures at baseline. SARP is a U.S.-based severe asthma cohort, so generalizability to populations outside the United States and to milder forms of asthma may be limited.
Conclusions
PSMD is an easily obtainable, reproducible marker of skeletal muscle adiposity and is lower (worse) in participants with asthma, and its baseline measurement predicts longitudinal lung function decline in women with asthma. Muscle adiposity as reflected as low attenuation on CT may be able to be improved with strength training. Future studies are needed to define the mechanistic insights among PSMD, markers of systemic inflammation and metabolic dysfunction, and lung function trajectory. Indeed, future studies are also needed to address whether consistent exercise-based programs in individuals with severe asthma can result in less lung function decline over time. Given the emerging role of metabolic disturbance in asthma, further investigations into the mechanisms of this association of PSMD and lung function decline are warranted.
Footnotes
Supported by the NHLBI to SARP III (Severe Asthma Research Program III) and American Heart Association Career Development Award (18CDA34110337) (M.C.T.). The following companies provided financial support for the SARP III study activities at the Coordinating and Clinical Centers beyond the third year of patient follow-up: AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Sanofi–Genzyme– Regeneron, and TEVA. These companies had no role in study design or data analysis, and the only restriction on the funds was that they be used to support the SARP initiative. Principal investigators, clinical centers, and data coordinating center as follows: U10 HL109164 (E.R.B., Deborah A. Meyers, and W.C.M.), U10 HL109257 (M.C.), U10 HL109250 (S.C.E.), U10 HL109146 (J.V.F.), U10 HL109250 (Benjamin Gaston), U10 HL109172 (E.I. and B.D.L.), U10 HL109168 (N.N.J.), U10 HL109250 (W. Gerald Teague), U10 HL109152 (S.E.W.), and U10 HL109086-04 (D.T.M.).
Author Contributions: Design: M.C.T., K.E.L., N.T., F.O., C.E.K., K.M.H., M.C.P., J.V.F., C.A.L., E.D., S.E.W., J.K.L., E.I., B.D.L., M.C., S.C.E., J.L., W.C.M., E.R.B., B.R.P., D.T.M., E.A.H., S.B.F., S.B.R., R.L.S., N.N.J., L.C.D., and M.L.S. Data acquisition: M.C.T., K.E.L., N.T., F.O., C.E.K., K.M.H., M.C.P., J.V.F., C.A.L., E.D., S.E.W., J.K.L., E.I., B.D.L., M.C., S.C.E., J.L., W.C.M., E.R.B., B.R.P., D.T.M., E.A.H., S.B.F., S.B.R., R.L.S., N.N.J., L.C.D., and M.L.S. Analysis: M.C.T., K.E.L., F.O., B.R.P., and D.T.M., Interpretation: M.C.T., K.E.L., N.T., F.O., C.E.K., K.M.H., M.C.P., J.V.F., C.A.L., E.D., S.E.W., J.K.L., E.I., B.D.L., M.C., S.C.E., J.L., W.C.M., E.R.B, B.R.P., D.T.M., E.A.H., S.B.F., S.B.R., R.L.S., N.N.J., L.C.D., and M.L.S.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202203-0597OC on October 4, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Hansen EF, Phanareth K, Laursen LC, Kok-Jensen A, Dirksen A. Reversible and irreversible airflow obstruction as predictor of overall mortality in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 1999;159:1267–1271. doi: 10.1164/ajrccm.159.4.9807121. [DOI] [PubMed] [Google Scholar]
- 2. Bai TR, Vonk JM, Postma DS, Boezen HM. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J . 2007;30:452–456. doi: 10.1183/09031936.00165106. [DOI] [PubMed] [Google Scholar]
- 3. Coumou H, Westerhof GA, de Nijs SB, Zwinderman AH, Bel EH. Predictors of accelerated decline in lung function in adult-onset asthma. Eur Respir J . 2018;51:1701785. doi: 10.1183/13993003.01785-2017. [DOI] [PubMed] [Google Scholar]
- 4. Vonk JM, Jongepier H, Panhuysen CI, Schouten JP, Bleecker ER, Postma DS. Risk factors associated with the presence of irreversible airflow limitation and reduced transfer coefficient in patients with asthma after 26 years of follow up. Thorax . 2003;58:322–327. doi: 10.1136/thorax.58.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dunican EM, Elicker BM, Gierada DS, Nagle SK, Schiebler ML, Newell JD, et al. National Heart Lung and Blood Institute (NHLBI) Severe Asthma Research Program (SARP) Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest . 2018;128:997–1009. doi: 10.1172/JCI95693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med . 1998;339:1194–1200. doi: 10.1056/NEJM199810223391703. [DOI] [PubMed] [Google Scholar]
- 7. Li X, Howard TD, Moore WC, Ampleford EJ, Li H, Busse WW, et al. Importance of hedgehog interacting protein and other lung function genes in asthma. J Allergy Clin Immunol . 2011;127:1457–1465. doi: 10.1016/j.jaci.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Denlinger LC, Phillips BR, Sorkness RL, Bleecker ER, Castro M, DeBoer MD, et al. Responsiveness to parenteral corticosteroids and lung function trajectory in adults with moderate-to-severe asthma. Am J Respir Crit Care Med . 2021;203:841–852. doi: 10.1164/rccm.202002-0454OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park CH, Yi Y, Do JG, Lee YT, Yoon KJ. Relationship between skeletal muscle mass and lung function in Korean adults without clinically apparent lung disease. Medicine (Baltimore) . 2018;97:e12281. doi: 10.1097/MD.0000000000012281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jones SE, Maddocks M, Kon SS, Canavan JL, Nolan CM, Clark AL, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax . 2015;70:213–218. doi: 10.1136/thoraxjnl-2014-206440. [DOI] [PubMed] [Google Scholar]
- 11. Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes . 1997;46:1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 12. Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985) . 2000;89:104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 13. Simoneau JA, Colberg SR, Thaete FL, Kelley DE. Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB J . 1995;9:273–278. [PubMed] [Google Scholar]
- 14. Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC study. J Appl Physiol (1985) . 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 15. Hu ZJ, He J, Zhao FD, Fang XQ, Zhou LN, Fan SW. An assessment of the intra- and inter-reliability of the lumbar paraspinal muscle parameters using CT scan and magnetic resonance imaging. Spine . 2011;36:E868–E874. doi: 10.1097/BRS.0b013e3181ef6b51. [DOI] [PubMed] [Google Scholar]
- 16. Keller A, Gunderson R, Reikerås O, Brox JI. Reliability of computed tomography measurements of paraspinal muscle cross-sectional area and density in patients with chronic low back pain. Spine . 2003;28:1455–1460. doi: 10.1097/01.BRS.0000067094.55003.AD. [DOI] [PubMed] [Google Scholar]
- 17. Therkelsen KE, Pedley A, Speliotes EK, Massaro JM, Murabito J, Hoffmann U, et al. Intramuscular fat and associations with metabolic risk factors in the Framingham Heart Study. Arterioscler Thromb Vasc Biol . 2013;33:863–870. doi: 10.1161/ATVBAHA.112.301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peters MC, Fahy JV. Metabolic consequences of obesity as an “outside in” mechanism of disease severity in asthma. Eur Respir J . 2016;48:291–293. doi: 10.1183/13993003.01132-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, et al. National Heart, Lung, and Blood Institute Severe Asthma Research Program Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med . 2016;4:574–584. doi: 10.1016/S2213-2600(16)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, et al. Baseline features of the Severe Asthma Research Program (SARP III) cohort: differences with age. J Allergy Clin Immunol Pract . 2018;6:545–554.e4. doi: 10.1016/j.jaip.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Phipatanakul W, Mauger DT, Sorkness RL, Gaffin JM, Holguin F, Woodruff PG, et al. Severe Asthma Research Program Effects of age and disease severity on systemic corticosteroid responses in asthma. Am J Respir Crit Care Med . 2017;195:1439–1448. doi: 10.1164/rccm.201607-1453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kramer H, Pickhardt PJ, Kliewer MA, Hernando D, Chen GH, Zagzebski JA, et al. Accuracy of liver fat quantification with advanced CT, MRI, and ultrasound techniques: prospective comparison with MR spectroscopy. AJR Am J Roentgenol . 2017;208:92–100. doi: 10.2214/AJR.16.16565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sieren JP, Newell JD, Jr, Barr RG, Bleecker ER, Burnette N, Carretta EE, et al. SPIROMICS Research Group SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med . 2016;194:794–806. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nattenmüller J, Schlett CL, Tsuchiya N, Reeder SB, Pickhardt PJ, Kramer H, et al. Representing the International Workshop for Pulmonary Functional Imaging (IWPFI) Noncontrast chest computed tomographic imaging of obesity and the metabolic syndrome: part II noncardiovascular findings. J Thorac Imaging . 2019;34:126–135. doi: 10.1097/RTI.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 25. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics . 1977;33:159–174. [PubMed] [Google Scholar]
- 26. Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known: 1916. Nutrition . 1989;5:303–311. [PubMed] [Google Scholar]
- 27. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J . 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Denlinger LC, Phillips BR, Ramratnam S, Ross K, Bhakta NR, Cardet JC, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program-3 Investigators Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med . 2017;195:302–313. doi: 10.1164/rccm.201602-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thiese MS, Ronna B, Ott U. P value interpretations and considerations. J Thorac Dis . 2016;8:E928–E931. doi: 10.21037/jtd.2016.08.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. STROBE Initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology . 2007;18:805–835. doi: 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- 31. Bidad N, Barnes N, Griffiths C, Horne R. Understanding patients’ perceptions of asthma control: a qualitative study. Eur Respir J . 2018;51:1701346. doi: 10.1183/13993003.01346-2017. [DOI] [PubMed] [Google Scholar]
- 32. Vähätalo I, Kankaanranta H, Tuomisto LE, Niemelä O, Lehtimäki L, Ilmarinen P. Long-term adherence to inhaled corticosteroids and asthma control in adult-onset asthma. ERJ Open Res . 2021;7:00715-2020. doi: 10.1183/23120541.00715-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Foer D, Beeler PE, Cui J, Karlson EW, Bates DW, Cahill KN. Asthma exacerbations in patients with type 2 diabetes and asthma on glucagon-like peptide-1 receptor agonists. Am J Respir Crit Care Med . 2021;203:831–840. doi: 10.1164/rccm.202004-0993OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med . 1990;322:223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- 35. Stump CS, Henriksen EJ, Wei Y, Sowers JR. The metabolic syndrome: role of skeletal muscle metabolism. Ann Med . 2006;38:389–402. doi: 10.1080/07853890600888413. [DOI] [PubMed] [Google Scholar]
- 36. Bonen A, Tan MH, Watson-Wright WM. Insulin binding and glucose uptake differences in rodent skeletal muscles. Diabetes . 1981;30:702–704. doi: 10.2337/diab.30.8.702. [DOI] [PubMed] [Google Scholar]
- 37.Komiya H, Mori Y, Yokose T, Kurokawa N, Horie N, Tajima N. Effect of intramuscular fat difference on glucose and insulin reaction in oral glucose tolerance test. J Atheroscler Thromb. 2006;13:136–142. doi: 10.5551/jat.13.136. [DOI] [PubMed] [Google Scholar]
- 38. Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes . 1994;43:1271–1278. doi: 10.2337/diab.43.11.1271. [DOI] [PubMed] [Google Scholar]
- 39. Forno E, Han YY, Muzumdar RH, Celedón JC. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J Allergy Clin Immunol . 2015;136:304–11.e8. doi: 10.1016/j.jaci.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yeh HC, Punjabi NM, Wang NY, Pankow JS, Duncan BB, Cox CE, et al. Cross-sectional and prospective study of lung function in adults with type 2 diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care . 2008;31:741–746. doi: 10.2337/dc07-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Forno E, Weiner DJ, Mullen J, Sawicki G, Kurland G, Han YY, et al. Obesity and airway dysanapsis in children with and without asthma. Am J Respir Crit Care Med . 2017;195:314–323. doi: 10.1164/rccm.201605-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cohen P, Noveral JP, Bhala A, Nunn SE, Herrick DJ, Grunstein MM. Leukotriene D4 facilitates airway smooth muscle cell proliferation via modulation of the IGF axis. Am J Physiol . 1995;269:L151–L157. doi: 10.1152/ajplung.1995.269.2.L151. [DOI] [PubMed] [Google Scholar]
- 43. Dekkers BG, Schaafsma D, Tran T, Zaagsma J, Meurs H. Insulin-induced laminin expression promotes a hypercontractile airway smooth muscle phenotype. Am J Respir Cell Mol Biol . 2009;41:494–504. doi: 10.1165/rcmb.2008-0251OC. [DOI] [PubMed] [Google Scholar]
- 44. Sipilä S, Suominen H. Effects of strength and endurance training on thigh and leg muscle mass and composition in elderly women. J Appl Physiol (1985) . 1995;78:334–340. doi: 10.1152/jappl.1995.78.1.334. [DOI] [PubMed] [Google Scholar]
- 45. Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC study. J Gerontol A Biol Sci Med Sci . 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 46. Tattersall MC, Guo M, Korcarz CE, Gepner AD, Kaufman JD, Liu KJ, et al. Asthma predicts cardiovascular disease events: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol . 2015;35:1520–1525. doi: 10.1161/ATVBAHA.115.305452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dyck DJ, Heigenhauser GJ, Bruce CR. The role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiol (Oxf) . 2006;186:5–16. doi: 10.1111/j.1748-1716.2005.01502.x. [DOI] [PubMed] [Google Scholar]