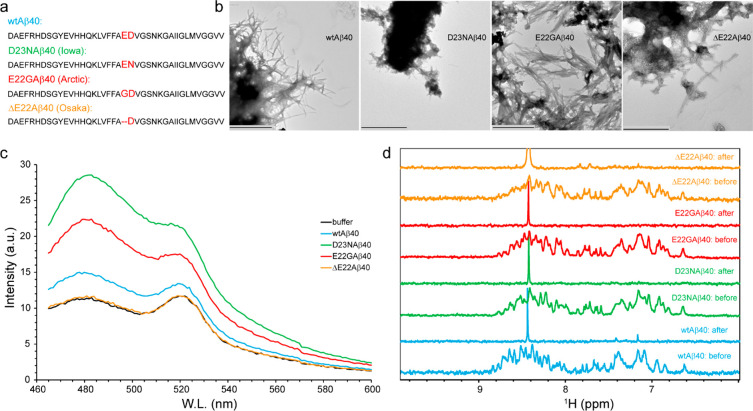
Figure 1.
Fibrillar or nonfibrillar aggregation of Aβ40 variants. (a) Amino acid sequence of wild-type-, D23N- (Iowa), E22G- (Arctic), and ΔE22-Aβ40 (Osaka) variants, with the site of mutation highlighted. (b,c) Transmission electron microscopy (TEM) images and Thioflavin T (ThT) fluorescence emission spectra of Aβ40 samples measured after 48 h of incubation in the aggregation condition (37 °C, gentle agitation). The wild-type-, D23N-, and E22G-Aβ40 formed ThT fluorescence-enhancing fibrils, while the ΔE22-Aβ40 variant showed fibrillar aggregation without ThT fluorescence enhancement. In (b), the scale bars represent 1000 nm for the wild-type and E22G and 600 nm for the D23N- and ΔE22-Aβ40. (d) 1D 1H NMR spectra of Aβ40 variants measured before and after incubation in the aggregation condition. The nearly complete loss of Aβ40 signals indicate conversion of Aβ40 monomers to slowly tumbling assemblies in all the studied Aβ40 variants.