Abstract
Objective:
To examine the relationship between duration of infant exposure to a moderate-to-large patent ductus arteriosus (PDA) shunt and the risk of developing bronchopulmonary dysplasia (BPD) or death before 36 weeks (BPD/Death).
Study Design:
Infants <28 weeks’ gestation who survived ≥7 days (n=423) had echocardiograms performed on day 7 and at planned intervals.
Results:
In multivariable regression models, BPD/Death did not appear to be increased until infants had been exposed to a moderate-to-large PDA for at least 7-13 days: OR (95%CI) (referent=closed or small PDA): moderate-to-large PDA exposure for <7 days: 0.38 (0.10-1.46); for 7-13 days= 2.12 (1.04-4.32); for ≥14 days=3.86 (2.15-6.96). Once the threshold of 7-13 days had been reached, additional exposure (≥14 days) did not significantly add to the increased incidence of BPD/Death: (referent exposure=7-13 days): exposure for 14-27 days=1.34 (0.52-3.45); for 28-48 days=2.34 (0.88-6.19); for ≥49 days=1.80 (0.59-5.47)). A similar relationship was found for the outcome of BPD-alone.
Conclusions:
Infants < 28 weeks’ gestation required at least 7-13 days of exposure to a moderate-to-large PDA before a significant increase in the incidence of BPD/Death was apparent. Once this threshold was reached additional exposure to a moderate-to-large PDA did not significantly add to the increased incidence of BPD/Death.
Keywords: ductus arteriosus, bronchopulmonary dysplasia, death, extremely low gestational age infants
Introduction:
Between 50-70% of infants <28 weeks gestational age (GA) have a patent ductus arteriosus (PDA) that persists for weeks after birth 1. Although there is an association between a PDA and the risk of developing bronchopulmonary dysplasia (BPD) or death before 36 weeks (BPD/Death), the risk appears to depend not on the presence or absence of a PDA but on the magnitude of the PDA shunt 2,3.
Whether the PDA plays a causative role in the development of BPD/Death is still unclear. Randomized controlled trials (RCTs) using prophylactic or early PDA treatment have not shown a decrease in the incidence of BPD/Death4–8. Nor has a decrease in the incidence of BPD/Death been found when PDA treatment begun after the first postnatal week was compared with an approach that delayed PDA treatment for an additional 2 weeks 9. Unfortunately, at the time these trials were designed no information was available to help investigators determine what duration of exposure to a moderate-to-large PDA might be needed before one could expect to see an increase in the risk of BPD/Death. Therefore, we designed the following observational study to examine the relationship between different durations of exposure to moderate-to-large PDA shunts that persist beyond the first postnatal week and the outcome BPD/Death. We hypothesized that there may be a critical length of PDA exposure that is necessary before a significant increase in the risk of BPD/Death becomes apparent.
Methods:
Patient Population and PDA treatment protocols:
The study was approved by the Institutional Review Board of the University of California San Francisco. Infants were included in the study population if they delivered before 280/7 weeks GA, were admitted to the intensive care nursery within 24 hours of birth between January 2005 and December 2018, and survived beyond 7 days.
All infants had an echocardiogram performed on postnatal day 7. The echocardiographic studies included two-dimensional imaging, M-mode, color flow mapping, and Doppler interrogation as previously described 10,11. A moderate-to-large PDA was defined by a ductus diameter ≥1.5mm plus one or more of the following echocardiographic criteria: A) left atrium-to-aortic root ratio ≥1.6; B) mean pressure gradient across the ductus ≤8mm; C) left pulmonary artery diastolic flow velocity >0.2 m/sec; and/or D) reversed diastolic flow in the descending aorta 10,11. Ductus that did not meet these criteria were considered to be “constricted” (small or closed).
Infants who had a moderate-to-large PDA on the echocardiogram performed on day 7 were followed with echocardiograms every 7 days for the next 2-3 weeks, then at least every other week until the PDA was no longer moderate-to-large in size. Infants with a “constricted” (small or closed) ductus on day 7 were examined daily for a change in clinical symptoms indicative of a reopened moderate-to-large PDA (systolic murmur or hyperdynamic precordium). If either of these occurred, an echocardiogram was performed within 24 hours. When a reopened moderate-to-large PDA was detected, echocardiograms were performed every 7 days for the next 2-3 weeks, and then at least every other week until the PDA was no longer moderate-to-large in size. Infants with a “constricted” ductus that never developed clinical signs of reopening had routine echocardiograms performed at least every 2-3 weeks to confirm ductus constriction until ductus closure or hospital discharge.
The duration of exposure to a moderate-to-large PDA that persisted beyond the first week was calculated and expressed in days. Infants with small or closed ductus at postnatal day 7 were assumed to have not been exposed to a moderate-to-large PDA during the first 7 days. Infants with moderate-to-large PDAs at postnatal day 7 were assumed to have been exposed to a moderate-to-large PDA for the entire 7 days. The time of ductus constriction was assigned as the halfway point between the last exam with a moderate-to-large PDA and the first exam with a constricted ductus. If infants died before an exam showed ductus constriction the ductus was assumed to be moderate-to-large at the time of death. When late reopening of the PDA occurred after documented ductus constriction, the additional exposure to the reopened moderate-to-large PDA shunt (calculated as the number of days from the echocardiogram demonstrating the reopened moderate-to-large shunt to the time of ductus constriction (i.e., the halfway point between the last exam with a moderate-to-large PDA and the first exam with a constricted ductus)) was added to the duration of any prior moderate-to-large PDA shunt exposure.
During the study period two different protocols for PDA treatment were used. Between January 2005 and May 2011, all infants <280/7 weeks GA were treated with a course of prophylactic indomethacin starting within 15 hours of birth 12. After May 2011, a more conservative PDA treatment approach was instituted: indomethacin was not given prophylactically, and pharmacologic treatment was delayed until at least postnatal day 8 to allow for spontaneous closure 13. During the conservative epoch, 54% of infants with moderate-to-large PDAs received pharmacologic treatment after the first week (indomethacin-alone=30%; acetaminophen (with indomethacin back-up)=24%); 46% were not treated because their respiratory requirements were stable or improving despite the moderate-to-large PDA shunt. Infants with moderate-to-large PDAs that failed to close after pharmacologic treatment were ligated only if the infants’ ventilatory support was escalating for 4-5 days, or failed to improve during a 2 weeks interval. During both epochs, “constricted” (small or closed) ductus were never treated.
There were no changes in our protocols for feeding advances or management of ventilation, fluids, and hypotension during the study period. Detailed descriptions of our approach to respiratory and hemodynamic support have been previously published 12.
A single neonatologist (RIC) prospectively evaluated and recorded all of the demographic factors and outcome measures during the hospitalization (see Table 1 for definitions). Our primary outcome was bronchopulmonary dysplasia or death before 36 weeks (BPD/Death). Bronchopulmonary dysplasia was defined using a modified room air challenge test between 360/7 and 366/7 weeks’ corrected age 14.
Table 1:
Demographic characteristics of infants who survived ≥7 days and were exposed to varying durations of moderate-to-large PDA shunts that persisted beyond the first week. Infants with small or closed ductus at postnatal day 7 were assumed to have not been exposed to a moderate-to-large PDA during the first 7 days. Infants with moderate-to-large PDAs at postnatal day 7 were assumed to have been exposed to a moderate-to-large PDA for the entire 7 days.
| Duration of exposure to a moderate-to-large PDA | |||||
|---|---|---|---|---|---|
| Variable | Closed/Small 1 | <7 days 2 | 7-13 days 3 | ≥14 days 4 | p-value |
| N= | 231 | 19 | 56 | 117 | |
| Prenatal Variables: | |||||
| Multiple Gestation - % | 29 | 37 | 39 | 36 | 0.310 |
| Preeclampsia - % | 23 | 16 | 27 | 23 | 0.808 |
| Maternal Diabetes - % | 11 | 21 | 11 | 16 | 0.383 |
| Chorioamnionitis - % | 23 | 58 | 16 | 15 | <0.001 |
| Betamethasone <24 hours - % | 26 | 26 | 36 | 26 | 0.498 |
| Caesarian Section - % | 66 | 58 | 70 | 72 | 0.560 |
| Neonatal Variables: | |||||
| Gestation-weeks (m±sd) 5 | 26.4±1.0 | 25.6±0.9 | 26.1±1.2 | 25.9±1.1 | <0.001 |
| Gestation ≤25 weeks - % | 31 | 63 | 48 | 49 | ≤0.001 |
| Birthweight – grams (m±sd) | 852±201 | 773±126 | 805±195 | 779±189 | 0.005 |
| Small for Gestational Age - % 6 | 12 | 0 | 9 | 13 | 0.678 |
| Outborn - % | 21 | 26 | 36 | 23 | 0.149 |
| Caucasian - % | 36 | 42 | 45 | 42 | 0.542 |
| Male - % | 51 | 42 | 50 | 44 | 0.592 |
| 5 minute Apgar ≤5 - % | 30 | 37 | 41 | 39 | 0.250 |
| Intubated during 1st 24 hours - % | 82 | 95 | 89 | 79 | 0.209 |
| Mechanical Ventilation at 24 hours - % | 52 | 74 | 73 | 66 | 0.005 |
| ICH (grades 3 or 4) - % 7 | 10 | 16 | 9 | 11 | 0.808 |
| Net Fluid Gain 1st 72 hours – ml (m±sd) 8 | 156±99 | 134±78 | 148±108 | 136±103 | 0.327 |
| Early Onset Infection - % 9 | 4 | 11 | 7 | 3 | 0.316 |
| Late Onset Infection - % 10 | 34 | 47 | 36 | 27 | 0.293 |
| Birth Epoch: (2005-2008/2009-2013/2014-2018) - % | 52/24/24 | 63/26/11 | 27/28/45 | 8/30/62 | <0.001 |
| Necrotizing Enterocolitis - % 11 | 14 | 11 | 14 | 17 | 0.768 |
| PDA Variables: | |||||
| Prophylactic Indomethacin - % | 73 | 79 | 29 | 21 | <0.001 |
| PDA Ligation - % | 0 | 58 | 23 | 18 | <0.001 |
| Any PDA Treatment - % 12 | 74 | 100 | 96 | 74 | <0.001 |
| PDA Reopened after 1st week - % 13 | 0 | 100 | 20 | 12 | <0.001 |
| Outcomes: | |||||
| BPD/Death - % 14 | 28 | 16 | 45 | 50 | <0.001 |
| BPD - % 15 | 23 | 16 | 33 | 47 | <0.001 |
| Death - % 16 | 9 | 0 | 20 | 7 | 0.052 |
Closed/Small, infants who either closed their ductus permanently during the first week or had a small PDA at the end of the first week that either closed or remained small throughout the hospitalization
<7 days, infants with constricted ductus during the first postnatal week (with small or closed ductus at postnatal day 7) who subsequently reopened their PDA after the initial ductus constriction and were exposed to the reopened moderate-to-large PDA shunt for <7 days
7-13 days, total exposure to a moderate-to-large PDA for 7-13 days: among infants who had a moderate-to-large PDA during the first week that persisted beyond 7 days and among infants with constricted ductus during the first week that later reopened, becoming moderate-to-large after the first week
≥14 days, total exposure to a moderate-to-large PDA for ≥14 days: among infants who had a moderate-to-large PDA during the first week that persisted beyond 7 days and among infants with constricted ductus during the first week that later reopened, becoming moderate-to-large after the first week
Gestation, gestational age was determined by the date of last menstrual period and early ultrasounds (before 24 weeks gestation)
Small for Gestational Age, Fenton birthweight-for-gestational-age z-scores <1.29 15
ICH (grades 3 or 4), serious intraventricular hemorrhages were defined as grades 3 or 4 intraventricular hemorrhage (using the four-level grading system) 16.
Net Fluid Gain 1st 72 hours, total fluid intake during first 3 days minus total urine output during first 3 days
Early Onset Infection, culture-positive bacteremia prior to 4 days of life
Late Onset Infection, culture-positive bacteremia or pneumonia after 3 days of life (pneumonia = sudden respiratory deterioration in arterial blood gases associated with a) new progressive infiltrates in the chest radiograph that persist for more than 3 days and b) either blood leukocytosis, leukopenia, or an increase in immature neutrophil forms, and/or c) associated temperature and/or glucose instability)
Necrotizing Enterocolitis, Bell’s classification II or greater (this included necrotizing enterocolitis that was treated medically or surgically, and “spontaneous perforations” that occurred before 10 days of life) 17.
Any PDA Treatment, infants who received prophylactic indomethacin and/or later pharmacologic PDA treatment
PDA Reopened after 1st week, ductus that were constricted during the first week, but then reopened after the first week developing a moderate-to-large shunt
BPD/Death, bronchopulmonary dysplasia or death before 36 weeks. Bronchopulmonary dysplasia was defined using a modified room air challenge test between 360/7 and 366/7 weeks’ corrected age 14
BPD, bronchopulmonary dysplasia: n=393 (Closed small (n=216), <7 days (n=19), 7-13 days (n=46), ≥14 days (n=112)): several infants died after 7 days and prior to completion of the room air challenge test at 36 weeks:
Death, death after 7 days and prior to hospital discharge
Statistical analysis
Chi-Squared, Fisher’s exact, and Student’s t-tests were used to compare groups for categorical and parametric variables. Our primary goal was to examine the relationship between different durations of PDA exposure and the combined outcome BPD/Death. Since several demographic variables that have the potential to affect BPD/Death differed among the PDA exposure groups (Table 1), we used multivariable logistic regression models to adjust for confounding. In addition, since the observational period of our study spanned a 14 years interval, we also examined the effects of birth epochs, when the infants were admitted to the nursery (2005-2008; 2009-2013; 2014-2018), on this relationship.
We first created a basic model for the outcome BPD/Death that included our variable of interest “duration of PDA exposure” and the variable “GA ≤ 25 weeks”. Using these two variables, we performed a logistic regression to determine the odds ratio (OR) of BPD/Death for the variable of interest “duration of PDA exposure”.
Next, we added each of the demographic or birth epoch variables listed in Table 1 to the basic model and re-ran the logistic regression to determine how much the OR for the variable “duration of PDA exposure” was altered by the addition of the new variable to the basic model. If the addition of the new variable altered the OR of the “duration of PDA exposure” by more than 10% it was considered to be an important demographic variable that should be added to the Final Adjusted model. We repeated this step for each of the demographic variables. Finally, we created the Final Adjusted model for the outcome BPD/Death by adding all of the important demographic variables to the basic model.
We used the same approach to create the Final Adjusted models for our secondary outcomes: BPD-alone and Death-alone.
Results:
During the study period 485 infants <280/7 weeks GA were admitted to the nursery. Sixty-two infants died before the end of the first week; the remaining 423 infants who survived beyond 7 days comprised our study population (Figure 1)
Figure:
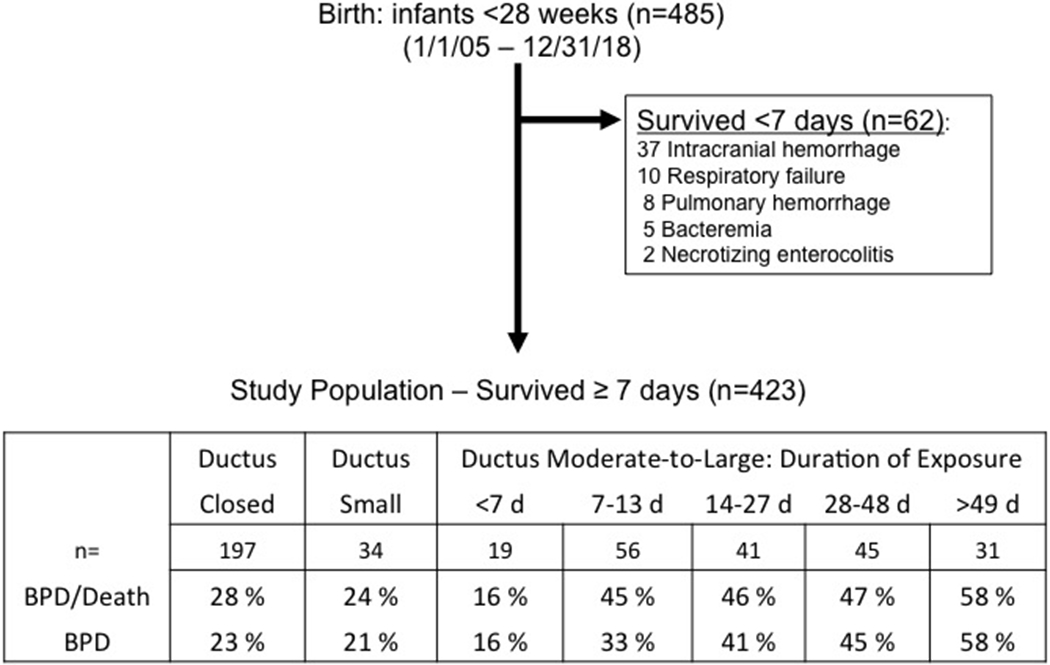
Flow diagram of patient distribution and outcomes.
Sixty-two infants died before entering the study: 37 from severe intracranial hemorrhage and redirection of care, 10 from severe respiratory distress syndrome, 8 from severe pulmonary hemorrhage, 5 from bacteremia, and 2 from necrotizing enterocolitis.
BPD (n=393): not all study infants lived long enough to complete the room air challenge test at 36 weeks.
Our primary goal was to examine the relationship between different durations of PDA exposure and the incidence of BPD/Death. We found no difference in the incidence of BPD/Death between infants who closed their ductus permanently during the first postnatal week and those with small PDAs at the end of the first week that either remained small throughout the hospitalization or closed before discharge (OR (95%CI): 0.77 (0.07-1.63)). On the other hand, infants with moderate-to-large PDAs that persisted beyond the first week had a significantly higher incidence of BPD/Death than those who closed their ductus during the first week (OR (95%CI): 2.04 (1.62-2.46)) (Figure 1).
We created multivariable models to examine the relationship between different durations of PDA exposure and the incidence of BPD/Death (Table 2) (see Methods). In the adjusted models, the incidence of BPD/Death did not appear to be increased until infants had been exposed to a moderate-to-large PDA for at least 7-13 days (Table 2: model 1). Once the infants had been exposed to a moderate-to-large shunt for 7-13 days, additional exposure (≥14 days) did not appreciably add to the already increased incidence of BPD/Death: PDA exposure for 7-13 days (referent: OR=1); 14-27 days (OR (95%CI)): 1.34 (0.52-3.45); 28-48 days: 2.34 (0.88-6.19); ≥49 days: 1.80 (0.59-5.47).
Table 2:
Adjusted risk models that examine the relationship between PDA exposure and the outcomes BPD/Death and BPD
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| Final Model | Final model + ligation | Final model + prophylactic indomethacin | Final model + any PDA Rx | Final model + PDA reopened | |
| Outcome: BPD/Death | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| PDA Exposure: | |||||
| Closed/small | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| <7 days | 0.38 (0.10-1.46) | 0.22 (0.05-1.05) | 0.37 (0.10-1.43) | 0.36 (0.09-1.39) | 1.40 (0.24-8.22) |
| 7-13 days | 2.12 (1.04-4.32) * | 1.77 (0.84-3.75) | 1.48 (0.68-3.23) | 2.00 (0.97-4.09) | 2.80 (1.29-6.08) * |
| ≥14 days | 3.86 (2.15-6.96) * | 3.36 (1.82-6.21) * | 2.73 (1.42-5.23) * | 3.95 (2.18-7.14) * | 4.63 (2.46-8.69) * |
| Gestation ≤25 weeks | 1.86 (1.11-3.12) * | 1.87 (1.11-3.13) * | 1.95 (1.16-3.29) * | 1.85 (1.11-3.11) * | 1.84 (1.09-3.09) * |
| Small for Gestation | 10.08 (3.88-26.21) * | 9.86 (3.79-25.64) * | 10.42 (4.05-26.82) * | 10.18 (3.86-26.81) * | 10.74 (4.08-28.26) * |
| Intubated during 1st day | 9.34 (3.61-24.12) * | 8.93 (3.45-23.15) * | 11.52 (4.32-30.69) * | 8.26 (3.14-21.74) * | 9.17 (3.50-24.00) * |
| Late Onset Sepsis | 4.08 (2.42-6.86) * | 3.94 (2.33-6.66) * | 4.68 (2.72-8.05) * | 3.95 (2.34-6.68) * | 4.49 (2.63-7.65) * |
| Net Fluid Gain during 1st 72 hours 1 | 1.002 (0.999-1.005) | 1.002 (0.999-1.005) | 1.003 * (1.000-1.005) | 1.002 (0.999-1.005) | 1.003 * (1.000-1.005) |
| PDA Ligation | 2.00 (0.825-4.85) | ||||
| Prophylactic Indomethacin | 0.49 (0.26-0.90) * | ||||
| Any PDA Rx | 1.49 (0.74-3.00) | ||||
| PDA Reopened | 0.27 (0.09-0.83) * | ||||
| Outcome: BPD | |||||
| PDA Exposure: | |||||
| Closed/small | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| <7 days | 0.36 (0.09-1.36) | 0.16 (0.48-2.49) * | 0.35 (0.09-1.32) | 0.32 (0.08-1.24) | 0.72 (0.13-4.01) |
| 7-13 days | 1.46 (0.67-3.14) | 1.10 (0.48-2.50) | 1.06 (0.46-2.44) | 1.31 (0.60-2.84) | 1.68 (0.74-3.78) |
| ≥14 days | 4.09 (2.32-7/22) * | 3.38 (1.87-6.10) * | 2.89 (1.50-5.59) * | 4.22 (2.37-7.51) * | 4.46 (2.43-8.18) * |
| Gestation ≤25 weeks | 1.84 (1.12-3.05) * | 1.84 (1.11-3.05) * | 1.89 (1.14-3.13) * | 1.85 (1.12-3.07) * | 1.83 (1.11-3.03) * |
| Intubated during 1st day | 14.18 (4.14-48.45) * | 13.27 (3.88-45.36) * | 16.47 (4.77-56.90) * | 11.85 (3.44-40.84) * | 13.87 (4.04-47.70) * |
| Late Onset Sepsis | 3.92 (2.32-6.63) * | 3.85 (2.27-6.55) * | 4.28 (2.50-7.33) * | 3.83 (2.26-6.50) * | 4.13 (2.42-7.05) * |
| PDA Ligation | 2.81 (1.14-6.92) * | ||||
| Prophylactic Indomethacin | 0.54 (0.29-0.99) * | ||||
| Any PDA Rx | 1.98 (0.95-4.14) | ||||
| PDA Reopened | 0.49 (0.17-1.46) |
p-value <0.05. OR, odds ratio. 95% CI, 95% confidence interval
Net Fluid Gain during 1st 72 hours, OR (95%CI) for 1 ml net fluid gain
The significant relationship between prolonged (≥14 days) PDA exposure and the incidence of BPD/Death appeared to persist even after adjusting for the types of PDA treatment infants received (prophylactic indomethacin, ligation, or any pharmacologic treatment (Table 2: models 2-4)) or the infants’ age when the persistent moderate-to-large shunt first appeared (i.e., whether it was present during the first postnatal week and persisted beyond 7 days or whether the ductus was constricted during the first postnatal week, and then reopened later, becoming moderate-to-large after the first week (Table 2: model 5)).
Increasing lengths of PDA exposure were also associated with the outcome “incidence of BPD alone” (Table 2). The incidence of BPD did not appear to be increased until infants had been exposed to a moderate-to-large PDA for at least 14 days (Table 2: model 1). Once the infants had been exposed to a moderate-to-large shunt for 14-27 days, additional exposure (≥28 days) did not significantly add to the already increased incidence of BPD: PDA exposure for 14-27 days (referent: OR=1); 28-48 days (OR (95%CI)): 2.02 (0.70-5.78); ≥49 days: 2.05 (0.68-6.18).
We found no consistent relationship between increasing lengths of PDA exposure and the incidence of death during the hospitalization (data not shown).
Discussion:
Recent studies have suggested that infants with small PDA shunts do not appear to be at increased risk for developing BPD/Death or BPD 2,3. Only infants with moderate-to-large PDA shunts appear to be at increased risk. Our results agree with the prior findings. While these results do not prove a cause–and-effect relationship, they do indicate that the presence of a moderate-to-large PDA may be a useful biomarker for identifying infants at increased risk for BPD/Death and BPD.
Our goal was to determine whether certain durations of PDA exposure are necessary before one might expect to see an increase in the incidence of BPD/Death and BPD. For both outcomes there appeared to be a minimal duration of exposure that was needed before a significant increase in risk could be detected (at least 7-13 days for BPD/Death; 14-27 days for BPD-alone). It is interesting to note that once these thresholds were reached further exposure was associated with only a modest, non-significant increase in risk for both outcomes. In our study the effects of prolonged PDA exposure appeared to be independent of whether the moderate-to-large shunt was present during the first week and continued to persist beyond 7 days or whether it first appeared after 7 days when a previously constricted ductus subsequently reopened (Table 2: model 5).
Our study has several limitations. As an observational study, it cannot distinguish between causation and association. The study also took place over a 14 years interval. Even though we examined the effects of being born during different birth epochs, or having different demographic variables, unmeasured differences in practice could have affected the rates of BPD/Death and BPD. We used data from a single center. Since the rates of moderate-to-large PDA vary widely by center 9 our results may not be generalizable to other centers where the rates of PDA differ from ours. The relatively small size of our study also may have made it difficult to detect significant differences among some of our PDA exposure subgroups. We focused our study on infants who continued to have a persistent PDA beyond the first week. Therefore, we cannot address whether brief moderate-to-large PDA exposures during the first week may have altered the study outcomes. Prior RCTs have examined this particular exposure group and have reported no noticeable effects on the incidence of BPD/Death and BPD 4–8.
Our study contains useful information for designing and powering future RCTs designed to examine the effects of different PDA treatments on the incidence of respiratory morbidity. We suggest that if a trial’s goal is to demonstrate whether closing the PDA makes a difference in the incidence of BPD/Death and BPD, then one needs to design a trial where one study group is exposed to a moderate-to-large shunt for <7days and the other is exposed to the shunt for >14 days. We speculate that one possible explanation for the failure of prior RCTs 4–9 to detect a causal relationship between PDA exposure and BPD/Death is that the durations of PDA exposure in the RCTs may not have been appropriate to detect a change in outcome. In the prior Prophylactic Treatment RCTs, the median difference in duration of exposure to a moderate-to-large PDA between the two treatment groups (“prophylactic treatment” and “conservative treatment”) was only 3 days4–8. Based on our analysis, this appears to be too short an interval to expect to see an increase in BPD/Death (Table 2). On the other hand, in the PDA-TOLERATE RCT, even though the median difference in PDA exposure between the two treatment groups (“early treatment” and “conservative treatment”) was 14 days, both treatment groups had prolonged moderate-to-large PDA exposures (median exposures were 16 days and 30 days, respectively) 9. Our study suggests that once infants have been exposed to moderate-to-large PDAs for ≥14 days, additional lengths of PDA exposure have little added impact on the incidence of BPD/Death.
Currently, there are several ongoing RCTs comparing the effects of PDA treatment or “non-treatment” starting at different times after birth (NCT01630278, NCT02884219, EudraCT:2013-005336-23, NCT02128191). The results of these trials should help to determine whether there is indeed a critical duration of exposure to a moderate-to-large PDA that plays an etiologic role in the development of BPD.
Acknowledgement:
We would like to thank Drs. Mark Cocalis, Laura Robertson, Michael Brook, Anita Moon-Grady and Shabnam Peyvandi for their expert help in reading and interpreting the echocardiograms. We would also like to thank the UCSF Clinical and Translational Science Institute for statistical consultation.
Financial Support:
This work was supported by a grant from the U.S. Public Health Service National Heart, Lung and Blood Institute (HL109199) and a gift from the Jamie and Bobby Gates Foundation.
Abbreviations:
- OR
odds ratio
- 95% CI
95% confidence interval
- BPD
bronchopulmonary dysplasia
- BPD/Death
bronchopulmonary dysplasia or death before 36 weeks
- Death
death after 7 days and prior to hospital discharge
- GA
gestational age
- NEC
necrotizing enterocolitis
- PDA
patent ductus arteriosus
- RCT
randomized controlled trial
References
- 1.Sung SI, Chang YS, Chun JY, et al. Mandatory Closure Versus Nonintervention for Patent Ductus Arteriosus in Very Preterm Infants. J Pediatr. 2016;177:66–71 [DOI] [PubMed] [Google Scholar]
- 2.Schena F, Francescato G, Cappelleri A, et al. Association between Hemodynamically Significant Patent Ductus Arteriosus and Bronchopulmonary Dysplasia. J Pediatr. 2015;166:1488–1492 [DOI] [PubMed] [Google Scholar]
- 3.Sellmer A, Bjerre JV, Schmidt MR, et al. Morbidity and mortality in preterm neonates with patent ductus arteriosus on day 3. Arch Dis Child Fetal Neonatal Ed. 2013;98:F505–510 [DOI] [PubMed] [Google Scholar]
- 4.Fowlie PW, Davis PG. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2010;7:CD000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooke L, Steer P, Woodgate P. Indomethacin for asymptomatic patent ductus arteriosus in preterm infants. Cochrane Database Syst Rev. 2003;2:CD003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst Rev. 2015;2:CD003481. [DOI] [PubMed] [Google Scholar]
- 7.Ohlsson A, Shah SS. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2011;7:CD004213. [DOI] [PubMed] [Google Scholar]
- 8.Benitz WE. Treatment of persistent patent ductus arteriosus in preterm infants: time to accept the null hypothesis? J Perinatol. 2010;30:241–252 [DOI] [PubMed] [Google Scholar]
- 9.Clyman RI, Liebowitz M, Kaempf J, et al. PDA-TOLERATE Trial: An Exploratory Randomized Controlled Trial of Treatment of Moderate-to-Large Patent Ductus Arteriosus at 1 Week of Age. J Pediatr. 2019;205:41–48 e46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jhaveri N, Moon-Grady A, Clyman RI. Early surgical ligation versus a conservative approach for management of patent ductus arteriosus that fails to close after indomethacin treatment. J Pediatr. 2010;157:381–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Hajjar M, Vaksmann G, Rakza T, Kongolo G, Storme L. Severity of the ductal shunt: a comparison of different markers. Arch Dis Child Fetal Neonatal Ed. 2005;90:F419–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liebowitz M, Clyman RI. Prophylactic Indomethacin Compared with Delayed Conservative Management of the Patent Ductus Arteriosus in Extremely Preterm Infants: Effects on Neonatal Outcomes. J Pediatr. 2017;187:119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch J, Hensley G, Roy L, Brown S, Ramaciotti C, Rosenfeld CR. Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics. 2006;117:1113–1121 [DOI] [PubMed] [Google Scholar]
- 14.Walsh MC, Yao Q, Gettner P, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114:1305–1311 [DOI] [PubMed] [Google Scholar]
- 15.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weight < 1500 grams. J. Pediatr. 1978;92:529–534 [DOI] [PubMed] [Google Scholar]
- 17.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]


