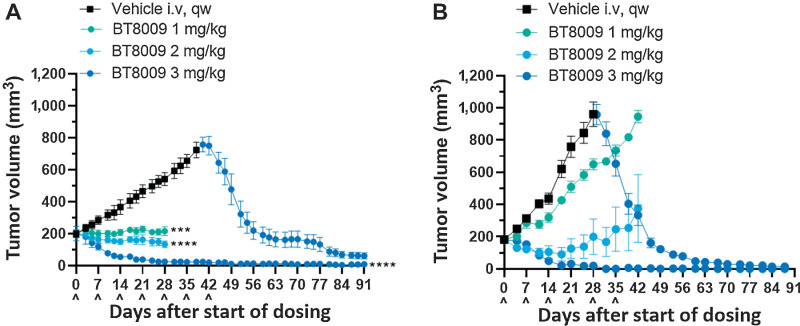
Figure 3.
BT8009 shows dose-related antitumor activity in; A, A CDX (triple-negative breast cancer) xenograft model. Dosing of the 3 mg/kg initial treatment group was ceased on day 42. Vehicle-treated animals were switched to 3 mg/kg BT8009 qw on day 40 and then 5 mg/kg qw on day 75. B, a PDX (non–small cell lung) xenograft model. Dosing of the 3 mg/kg initial treatment group was ceased on day 45. Vehicle-treated animals were switched to 3 mg/kg BT8009 qw on day 36. Tumor volumes are shown as mean ± standard error of the mean (n = 3–5) and statistical analysis performed with ordinary one-way ANOVA with Tukey's post hoc test for multiple comparisons ***, P < 0.001 and ****, P < 0.0001.