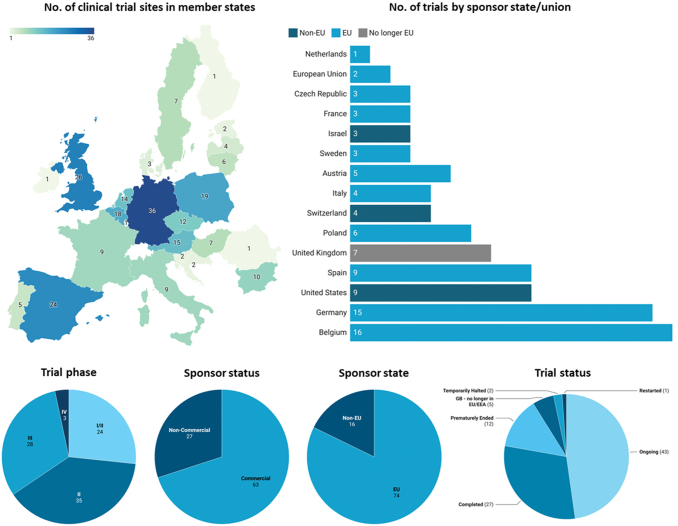
FIG. 1.
Geographical overview of clinical trial activity investigating TEPs in each Member State according to the EU Clinical Trials register, analyzed on January 22, 2022. The geographical map represents all trial sites across EU states, where some trials contain multiple sites across states. “No. of trials by sponsor state/union” represents the number of individual trials funded by a sponsor in each named jurisdiction. Sponsor state, trial phase, sponsor status, and trial status reflect individual trials with unique EudraCT numbers. Funding sources for TEP clinical trials are divided into sponsors located in EU and non-EU states. Two trials sponsored by the EU through the European Commission and Horizon 2020 listed the EU as the sponsor and thus no state is given. However, while UK is no longer in the EU, it has been included in this graph to accurately represent clinical activity over the last 12 years. Trial-phase status is based on the most recent updated list in the EU Clinical Trials Register. Phase I trials are not publicly available in the EudraCT database, except those including pediatric populations. “Other” in trial status includes two halted trials and one restarted trial. EU, European Union; TEP, tissue-engineered product; UK, United Kingdom. Color images are available online.