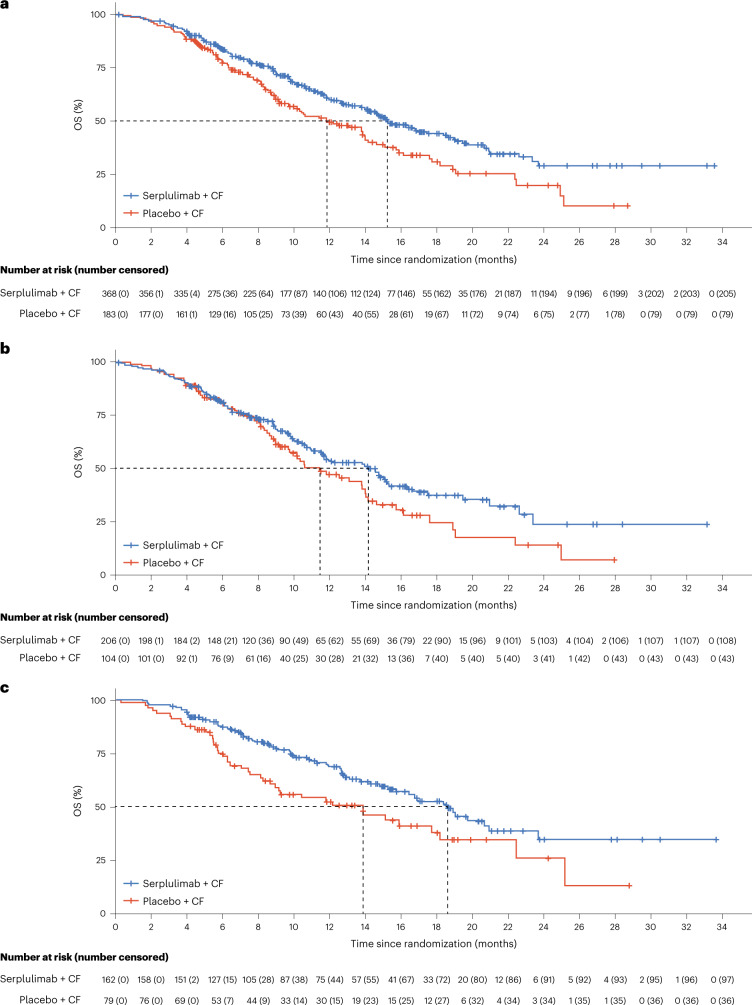
Fig. 3. Kaplan–Meier estimates of OS.
a, All randomized patients. For serplulimab + CF, n = 368, median OS 15.3 months (95% CI, 14.0–18.6 months). For placebo + CF, n = 183, median OS 11.8 months (95% CI, 9.7–14.0 months). HR, 0.68; 95% CI, 0.53–0.87; P = 0.0020. b, Patients with PD-L1 expression level of 1 ≤ CPS < 10. For serplulimab + CF, n = 206, median OS 14.2 months (95% CI, 11.5–15.3 months). For placebo + CF, n = 104, median OS 11.4 months (95% CI, 9.2–14.0 months). HR, 0.74; 95% CI, 0.54–1.03; P = 0.066. c, Patients with PD-L1 CPS ≥ 10. For serplulimab + CF, n = 162, median OS 18.6 months (95% CI, 15.3–20.9 months). For placebo + CF, n = 79, median OS 13.9 months (95% CI, 8.3–18.2 months). HR, 0.59; 95% CI, 0.40–0.88; P = 0.0082. Tick marks, data censored on the last known survival date.