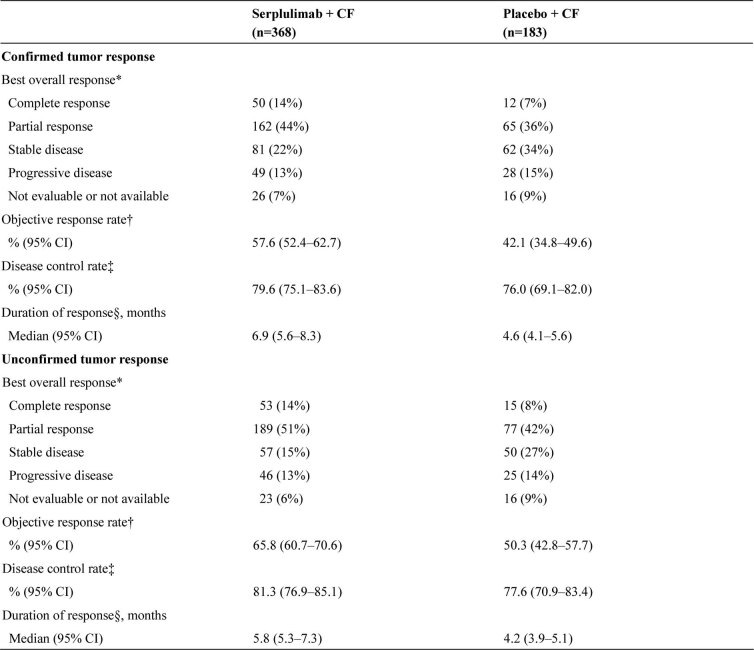
Extended Data Table 1.
Antitumor response assessed by independent radiological review committee in the ITT population
Data are n (%) unless otherwise stated. Antitumor response was assessed per the Response Evaluation Criteria in Solid Tumors version 1.1. CF = cisplatin and 5-fluorouracil. CI = confidence interval. *Percentages may not add up to 100% due to rounding. †Objective response rate was defined as proportion of patients achieving complete or partial response. ‡Disease control rate was defined as proportion of patients achieving complete or partial response, or stable disease. §Duration of response was assessed in patients who achieved complete or partial response and defined as time from first objective response to disease progression or death from any cause.