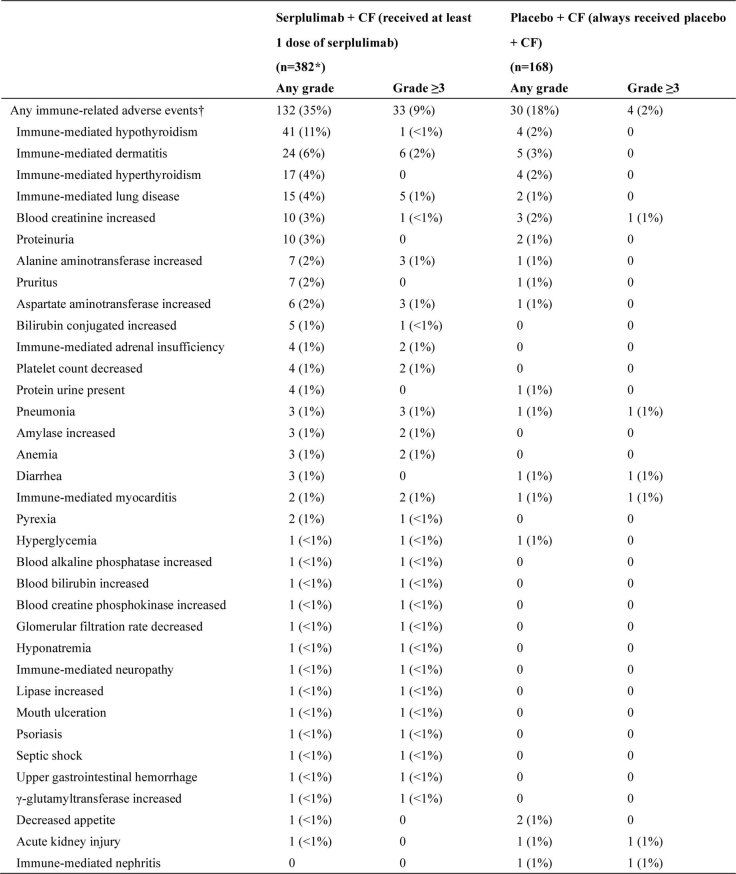
Extended Data Table 5.
Immune-related adverse events
Data are n (%). Immune-related adverse events of any grade occurring in ≥1% of patients in either group or any immune-related adverse events of grade ≥3 are shown. Six (2%) patients receiving serplulimab plus chemotherapy reported grade 5 immune-related adverse events (two reported immune-mediated lung disease, two reported immune-mediated myocarditis, one reported immune-mediated lung disease and septic shock, and one reported immune-mediated dermatitis). No grade 5 immune-related adverse event occurred in patients receiving placebo plus chemotherapy. Adverse events were recorded from the first dose of study treatment until 90 days after the last dose or the start of new antitumor treatment, whichever occurred first. CF = cisplatin and 5-fluorouracil. *Inclusive of 15 patients who were randomized to receive placebo plus chemotherapy, but received serplulimab plus chemotherapy due to an error in drug distribution. †Immune-related adverse events were defined as adverse events that were associated with drug exposure and demonstrated immune-mediated mechanisms with no other unequivocal etiology.