Abstract
Disparities in premature cardiovascular mortality (PCVM) have been associated with socioeconomic, behavioral, and environmental risk factors. Understanding the “phenotypes”, or combinations of characteristics associated with the highest risk of PCVM, and the geographic distributions of these phenotypes is critical to targeting PCVM interventions. This study applied the classification and regression tree (CART) to identify county phenotypes of PCVM and geographic information systems to examine the distributions of identified phenotypes. Random forest analysis was applied to evaluate the relative importance of risk factors associated with PCVM. The CART analysis identified seven county phenotypes of PCVM, where high-risk phenotypes were characterized by having greater percentages of people with lower income, higher physical inactivity, and higher food insecurity. These high-risk phenotypes were mostly concentrated in the Black Belt of the American South and the Appalachian region. The random forest analysis identified additional important risk factors associated with PCVM, including broadband access, smoking, receipt of Supplemental Nutrition Assistance Program benefits, and educational attainment. Our study demonstrates the use of machine learning approaches in characterizing community-level phenotypes of PCVM. Interventions to reduce PCVM should be tailored according to these phenotypes in corresponding geographic areas.
Subject terms: Epidemiology, Cardiology, Risk factors, Health care, Disease prevention, Health services, Public health, Cardiovascular diseases
Introduction
Premature cardiovascular mortality (PCVM) remains the leading cause of death in people under 65 years of age in the United States1. Although cardiovascular mortality rates in the overall population have declined steadily over the past decades, recent evidence shows that improvements in younger individuals have plateaued2,3. Additional evidence suggests that the burden of cardiovascular disease (CVD) mortality is unevenly distributed across geographic areas4. There is a substantial geographical variation in CVD mortality across US counties, most pronounced for ischemic heart disease and cerebrovascular disease4. Similar trends have also been observed for PCVM5.
Previous studies have linked socioeconomic, behavioral, and environmental risk factors to PCVM outcomes using composite metrics and indices, such as the social vulnerability and the sociodemographic indices6,7. However, to our knowledge, no prior study has investigated the community-level phenotypes of PCVM, or the combinations of risk factors highly associated with PCVM, as well as their geographic distributions. In this study, we identified county phenotypes of PCVM in the US from a broad range of risk factors using novel machine learning approaches. By presenting these phenotypes on a map, we were able to locate areas of high PCVM risk and their associated characteristics. We additionally compared the relative importance of risk factors in predicting PCVM.
Identifying not only the clusters of high PCVM but also the clusters of PCVM phenotypes acknowledges the complex relationships among selected drivers of high PCVM and PCVM disparities. This place-specific phenotype approach offers researchers and practitioners a framework for addressing community-level disparities in PCVM.
Methods
Study population
Our study population included individuals aged 15–64 years who died from CVD during the years 2015–2019 in the contiguous United States. PCVM was defined as the number of deaths in persons aged 15–64 years caused by CVD per 100,000 people at the county level, age-adjusted to the 2000 US Standard Population. We only included counties with at least 20 deaths from CVD during the study period to mitigate against unstable PCVM estimates. Counties from Hawaii and Alaska were excluded from the analysis due to the lack of complete risk factor data.
Data sources
Mortality data were accessed through the multiple cause of death files, maintained by the National Center for Health Statistics via the Centers for Disease Control and Prevention Wide-ranging Online Data for Epidemiologic Research (CDC-WONDER) database8. This database contains death certificates data from all fifty states, with cause of death identified by the international classification of disease, version 10 (ICD-10) coding schema. Data from the CDC-WONDER also include age at death, sex, race, and county of death. If multiple underlying causes of death on the death certificate are noted, a single cause is inserted according to the sequence of conditions on the certificate and contributing causes of death according to prespecified methods8. ICD-10 codes for CVD mortality were defined as follows: ischemic heart disease (I20–I25), heart failure (I50), cerebrovascular diseases (I60–I69), and hypertensive heart disease (I10–I15).
County-level risk factor data were harvested from a variety of data sources (Table 1), including County Health Rankings & Roadmaps9, Area Health Resources Files10, and Environmental Protection Agency’s Environmental Justice Screening tool (EJSCREEN)11. To best align temporally with the PCVM data, we used risk factor data collected in 2017 (the mid-year of the PCVM data) or the year closest to 2017. We used the 2020 EJSCREEN data (covering the years 2014–2020), and we re-estimated county-level exposures using the method outlined by the EPA EJSCREEN technical documentation guide since EJSCREEN data is natively reported at the census block group level11. We also visualized the geographic distributions of all county-level risk factors used in the study (Supplemental Fig. S1).
Table 1.
Definition and summary statistics of risk factors in the study.
| Variables | Description | Year | Original source | Mean (SD) |
|---|---|---|---|---|
| Race/ethnicity | ||||
| Hispanic | Percentage of population identifying as Hispanics | 2017 | Census—PE | 9.2 (13.3) |
| Non-hispanic black | Percentage of Non-Hispanic African American population | 2017 | Census—PE | 10.6 (15.1) |
| Non-hispanic white | Percentage of Non-Hispanic White population | 2017 | Census—PE | 75.4 (19.8) |
| Asian and Pacific Islander | Percentage of Asian and Pacific Islander population | 2017 | Census—PE | 1.7 (2.7) |
| Population structure | ||||
| Population age ≤ 18 | Percentage of population age 18 years or younger | 2017 | Census—PE | 22.2 (3.2) |
| Population age 65 + | Percentage of population age 65 years or older | 2017 | Census—PE | 18.2 (4.2) |
| Population female | Percentage of female population | 2017 | Census—PE | 50.2 (2.0) |
| Rural population | Percentage of people living in rural areas | 2010 | Census—PE | 52.4 (29.5) |
| Environmental exposure | ||||
| PM2.5 level in air | PM2.5 levels in air, µg/m3 (annual average) | 2018 | EPA | 8.0 (1.2) |
| Air toxics respiratory hazard index | Ratio of exposure concentration to health-based reference concentration | 2017 | EPA | 0.39 (0.14) |
| Ozone level in air | Ozone summer seasonal average of daily maximum 8 h concentration in air in parts per billion | 2018 | EPA | 41.5 (4.9) |
| Diesel PM level in air | Diesel particulate matter level in air, µg/m3 | 2017 | EPA | 0.24 (0.16) |
| Traffic proximity and volume | Average annual daily count of vehicles at major roads within 500 m, divided by distance in meters | 2019 | EPA | 181.2 (318.1) |
| Pre-1960 housing | Fraction of housing units built pre-1960, as indicator of potential lead paint exposure | 2016–2020 | Census—ACS | 0.27 (0.14) |
| Proximity to RMP sites | Count of RMP (potential chemical accident management plan) facilities within 5 km (or nearest one beyond 5 km), each divided by distance in km | 2020 | EPA | 0.49 (0.48) |
| Proximity to hazardous waste facilities | Count of hazardous waste facilities within 5 km (or nearest beyond 5 km), each divided by distance in km | 2020 | EPA | 0.84 (5.23) |
| Proximity to NPL sites | Count of proposed or listed NPL—also known as superfund—sites within 5 km (or nearest one beyond 5 km), each divided by distance in km | 2020 | EPA | 0.07 (0.10) |
| Major dischargers to water indicator | Modeled Toxic Concentrations at stream segments within 500 m, divided by distance in km | 2019 | EPA | 16.2 (622.2) |
| Socioeconomic status | ||||
| Income inequality | Ratio of household income at the 80th percentile to income at the 20th percentile | 2015–2019 | Census—ACS | 4.6 (0.7) |
| High school degree | Percentage of people (aged ≥ 25 year) and over with a high school diploma or equivalent | 2015–2019 | Census—ACS | 86.5 (5.9) |
| College degree | Percentage of people (aged 25–44 year) with some post-secondary education | 2015–2019 | Census—ACS | 57.5 (11.4) |
| Unemployment | Percentage of people (aged ≥ 16 year) unemployed but seeking work | 2017 | BLS | 4.8 (1.5) |
| Median Household Income | The income (US dollar) where half of households in a county earn more and half of households earn less | 2017 | AHRF | 51,231 (14,030) |
| Poverty | Percentage of people whose income under the federal poverty level | 2017 | AHRF | 15.8 (6.3) |
| Under 200% poverty | Percentage of people (aged 18–64 year) whose income is under 200% of the federal poverty level | 2017 | AHRF | 33.3 (9.2) |
| Receipt of SNAP benefits | Percentage of people who were food stamp recipients | 2017 | AHRF | 14.3 (6.8) |
| Not proficient in English | Percentage of people (aged ≥ 5 year) who reported speaking English less than very well | 2015–2019 | Census—ACS | 1.7 (2.6) |
| Severe housing problems | Percentage of households with at least 1 of 4 housing problems: overcrowding, high housing costs, lack of kitchen facilities, or lack of plumbing facilities | 2013–2017 | CHAS | 14.0 (3.8) |
| Severe housing cost burden | Percentage of households that spend 50% or more of their household income on housing | 2015–2019 | Census—ACS | 11.4 (3.3) |
| Homeownership | Percentage of owner-occupied housing units | 2015–2019 | Census—ACS | 71.1 (8.2) |
| Broadband access | Percentage of households with broadband internet connection | 2015–2019 | Census—ACS | 75.7 (8.9) |
| Social associations | Number of membership associations per 10,000 population | 2017 | CBP | 11.2 (4.3) |
| Health status | ||||
| Diabetes | Percentage of adults (age ≥ 20) with diagnosed diabetes (age-adjusted) | 2017 | CDC—DSS | 12.7 (3.7) |
| Low birthweight | Percentage of live births with low birthweight (< 2500 g) | 2013–2019 | CDC—NCHS | 8.4 (1.9) |
| Sexually transmitted infections | Number of newly diagnosed chlamydia cases per 100,000 people | 2017 | CDC—NCHHSTP | 419.1 (246.5) |
| Health behavior | ||||
| Adult obesity | Percentage of the adult population (aged ≥ 18 year) that reports a body mass index ≥ 30 (age-adjusted) | 2017 | CDC—DSS | 34.0 (5.8) |
| Insufficient sleep | Percentage of adults who report fewer than 7 h of sleep on average (age-adjusted) | 2018 | CDC—BRFSS | 37.5 (3.8) |
| Excessive drinking | Percentage of adults reporting binge or heavy drinking (age-adjusted) | 2017 | CDC—BRFSS | 17.4 (3.2) |
| Adult smoking | Percentage of adults who are current smokers (age-adjusted) | 2017 | CDC—BRFSS | 17.9 (3.5) |
| Physical inactivity | Percentage of adults (age ≥ 18 year) reporting no leisure-time physical activity (age-adjusted) | 2017 | CDC—DSS | 27.1 (6.0) |
| Flu vaccinations | Percentage of fee-for-service Medicare enrollees that had an annual flu vaccination | 2017 | CMS—MMD | 43.5 (8.5) |
| Food insecurity | Percentage of people who lack adequate access to food | 2017 | MMG | 13.6 (4.0) |
| Limited access to healthy foods | Percentage of people who are low-income and do not live close to a grocery store | 2015 | USDA—FEA | 7.2 (5.5) |
| Access to exercise opportunities | Percentage of people with adequate access to locations for physical activity | 2010 & 2019 | ESRI & Census—TF | 64.9 (21.8) |
| Driving alone to work | Percentage of the workforce that drives alone to work (indicators of physical inactivity and the transit system) | 2015–2019 | Census—ACS | 81.1 (5.8) |
| Long commute-driving alone | Among workers who commute in their car alone, the percentage that commute more than 30 min (indicator of physical inactivity) | 2015–2019 | Census—ACS | 33.5 (12.1) |
| Clinical care | ||||
| Uninsured rate | Percentage of people (aged 18–64 year) without health insurance | 2017 | HRSA—AHRF | 13.2 (6.0) |
| Primary care physicians | Primary care physicians in patient care per 100,000 people | 2017 | HRSA—AHRF | 53.7 (33.0) |
| Hospitals | Hospitals per 100,000 people | 2019 | HRSA—AHRF | 3.5 (3.5) |
| Community health centers | Community health centers per 100,000 people | 2017 | HRSA—AHRF | 5.5 (9.1) |
Variables obtained from AHRF: all variables under Clinical Care, Receipt of SNAP benefits, Median Household Income, Poverty, Under 200% poverty; Variables obtained from EPA-EJSCREEN: all variables under Environmental Exposure; Variables obtained from CHR: all other variables.
AHRF area health resources files, BLS bureau of labor statistics, BRFSS behavioral risk factor surveillance system, CBP county business patterns, CDC centers for disease control and prevention, CHAS comprehensive housing affordability strategy, CHF county health rankings & roadmaps, CMS centers for medicare & medicaid services, DSS US diabetes surveillance system, EJSCREEN environmental justice screening tool, EPA environmental protection agency, FEA food environment atlas, HRSA health resources and services administration, MMD mapping medicare disparities (MMD) tool, MMG map the meal gap, NCHHSTP national center for HIV/AIDS, viral hepatitis, STD, and TB prevention, NCHS national center for health statistics, NLP national priorities list, PE population estimates, PM fine particulate matter, RMP risk management plan, SNAP supplemental nutrition assistance program, TF tigerline files, USDA US department of agriculture.
Given the deidentified nature of the data and no individual-level data was used, institutional review board approval was not required.
Statistical analysis
We applied CART and random forest machine learning methods and geographic information systems to explore the association between county-level risk factors and PCVM. CART was used to identify phenotypes of PCVM, or combinations of county-level characteristics that were associated with PCVM12. We performed additional analyses to examine whether the county-level mortality rates for each subtype of PCVM (i.e., heart failure, hypertension, ischemic heart disease, and stroke) have a similar pattern after group them according to the phenotypes identified by the main model. Finally, we used random forest analysis13 to examine the relative importance of risk factors in predicting PCVM. We compared the concordance between the CART and random forest models, with a key focus on whether high-importance variables from the random forest models were included in the phenotypes identified by the CART analysis.
CART uses conditional inference to recursively partition data into smaller and homogeneous groups characterized by combinations of predictors14,15. At each split, the data are divided into two groups by an algorithm-selected variable and a threshold value that maximizes the difference between the split groups. The splitting procedure recursively repeats for each split group until some user-defined stopping criteria are met. We set the following stopping criteria: a maximum tree depth of six splits, a minimum number of 200 counties in a terminal node, and a statistical significance for variable splits (α < 0.05) using the Pearson correlation test. Each terminal node of the tree consists of a group of counties with similar levels of PCVM. The combination of characteristics associated with a terminal node represents a phenotype of PCVM. We then used geographic information systems to visualize the distribution of the identified phenotypes.
The CART models were established using a randomly sampled training set (consisting of 80% of all counties) and the results were validated against the test set (consisting of the rest 20% of the counties). To validate the results and the reproducibility of the CART model, we performed sensitivity analyses using three additional random samples as the training set and compared the results with the main model. We also conducted a sensitivity analysis of the CART approach with a different minimum number of counties (100) in a terminal node.
In contrast to CART which relies on only one tree, random forest creates and aggregates an ensemble of trees using random variable selection and bootstrap sampling13. It then takes an average of the outputs of these trees as a prediction. Next, the mean decrease in node impurity is used to calculate variables’ relative importance in predicting the outcome. We created 20,000 trees incorporating all risk factors as predictors. The number of variables randomly sampled as candidates at each tree split was set to 5.
SAS v9.4 was used for data management activities. R v3.6.1 was used for the machine learning analyses (packages “partykit”—ctree for CART and “randomForest” for random forest). Python 3.10.6 (packages “geopandas” and “matplotlib”) was used for maps in Figs. 2, S1. ArcGIS Pro v2.7.0 was used for maps in Fig. 3.
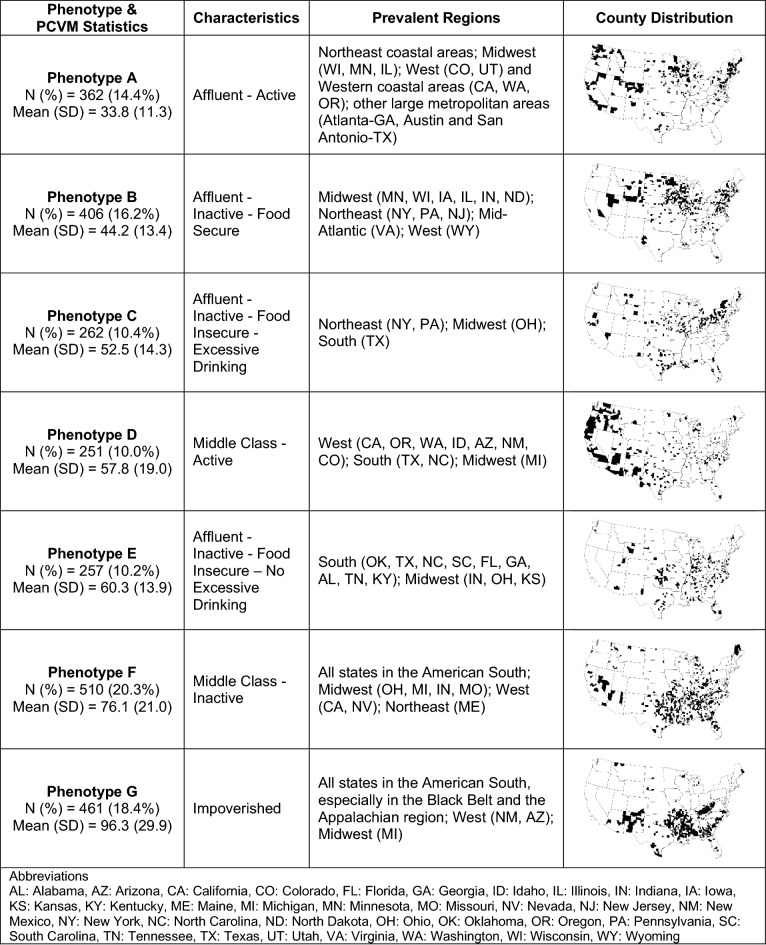
Figure 2.
Characteristics of county premature cardiovascular mortality (PCVM) phenotypes identified by CART. Notes: Counties in training and test sets were both included. Maps were created by Python v3.10.6 (https://www.python.org/) and its libraries: geopandas (v0.11.1) and matplotlib (v3.5.3).
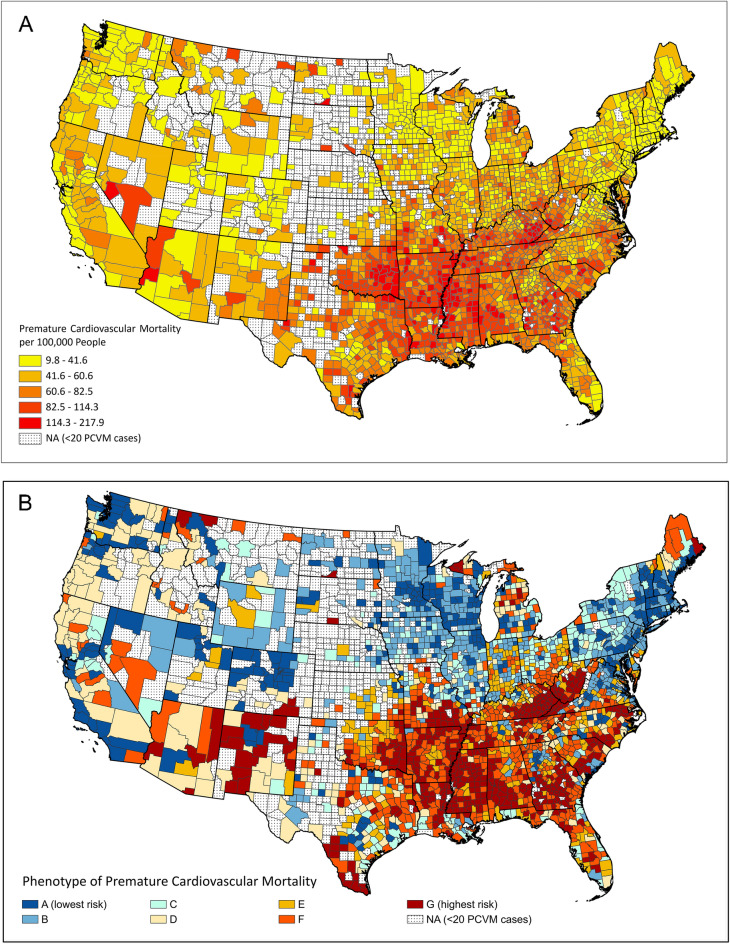
Figure 3.
US County Maps of (A) age-adjusted premature cardiovascular mortality (per 100,000 people), and (B) county phenotypes of premature cardiovascular mortality. Note: maps were created by ArcGIS Pro v2.7.0 (https://pro.arcgis.com/).
Results
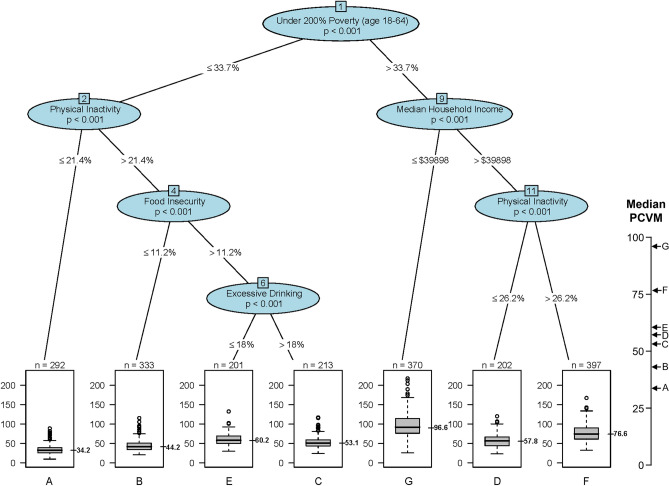
The study included 2509 counties, representing a total of 604,810 deaths from PCVM. There were 2008 and 501 randomly sampled counties in the training set and the test set, respectively. The baseline county characteristics were similar between the training and test sets as shown in Supplemental Table S1. The CART analysis identified seven phenotypes (A to G, in ascending order of the median PCVM) using the training dataset (n = 2008) (Fig. 1). The algorithm selected five variables from all candidate predictors serving as the six splitting nodes in the outcome tree, with under 200% of poverty at the top of the tree followed sequentially by physical inactivity, median household income, food insecurity, physical inactivity, and excessive drinking. All splits were statistically significant (p < 0.001).
Figure 1.
Classification and regression tree analysis (200 minimum counties at a terminal node) to predict county-level premature cardiovascular mortality (PCVM) using counties in the training set (N = 2008). Notes: Each path down to a terminal node represents a county phenotype. Box plots in the terminal nodes represent age-adjusted PCVM (per 100,000 people).
Applying the CART model to the test dataset showed no substantial differences in the PCVM distributions versus the training dataset (Supplementary Fig. S2). We summarized the statistics, characteristics, as well as geographic distribution of the identified phenotypes in Fig. 2, including counties in both training and test sets.
On the right side of the tree (Fig. 1), phenotype G (Impoverished) had the highest median PCVM (96.6) among all phenotypes, consisting of counties with more people (aged 18–64) under 200% of the federal poverty level (> 33.7%) and a lower median household income (≤ $39,898). Compared to phenotype G counties, counties of both phenotypes D (Middle Class—Active) and F (Middle Class—Inactive) had a lower median PCVM. Phenotype F counties differentiated from those of Phenotype D by having more people who were physically inactive.
On the left side of the tree (Fig. 1), all counties had fewer people (aged 18–64) under 200% of the federal poverty level and generally had lower rates of PCVM (except for phenotype E counties). Phenotype A (Affluent—Active), with a lower physical inactivity rate (≤ 21.4%), had the lowest median PCVM (34.2), about a third of the median PCVM for phenotype G (96.6). With more people who were physically inactive, phenotypes B (Affluent—Inactive—Food Secure), C (Affluent—Inactive—Food Insecure—Excessive Drinking), and E (Affluent—Inactive—Food Insecure—No Excessive Drinking) also had a higher median PCVM compared to phenotype A. Food insecurity further distinguished phenotype B with C and E, where phenotype B had fewer people who lack adequate access to food (≤ 11.2%) and had about 9 to 16 fewer deaths from CVD per 100,000 people compared to phenotypes C and E. Excessive drinking further separated phenotypes C and E, where phenotype C had more adults reporting binge or heavy drinking and a slightly lower median PCVM compared to phenotype E (53.1 vs. 60.2).
We calculated the county-level PCVM rates of each CVD subtype and grouped counties according to the phenotypes identified by the main model. Supplementary Fig. S3 shows that, for each subtype of PCVM, the median rates of PCVM grouped by phenotype were in ascending order from phenotype A to G, which is consistent with the main model.
The results of the sensitivity analysis of the CART model using three additional samples of counties as the training set were shown in Supplementary Fig. S4. We noticed that the top three nodes (under 200% poverty, physical inactivity, and median household income) in the additional models were the same as in the main model in Fig. 1, despite their splitting values being slightly different. In all four models, physical inactivity was the next splitting node after median household income. Food insecurity and excessive drinking, two variables that were present in the main model, appeared once and not at all, respectively, in the additional models. Poverty, a variable not presented in the main model, was present in all additional models. The results of the sensitivity analysis suggest that CART was relatively stable to changes in data structure, especially for the top splitting variables.
The sensitivity analysis of the CART model with a minimum number of 100 counties in a terminal node included more splitting nodes as well as more phenotypes in the model output (Supplementary Fig. S5), suggesting that additional risk factors were significantly associated with county-level PCVM in different subgroups of the population. These additional splitting variables included broadband access, uninsured (age 18–64), smoking, and receipt of Supplemental Nutrition Assistance Program (SNAP) benefits. Supplementary Fig. S6 illustrates the CART model applied to the test dataset, which revealed no significant differences compared to the model derived from the training dataset.
Figure 3A,B present the geographic distributions of the county-level PCVM and the phenotypes (for counties in both the training and test sets) from the main model. We observed that counties with high PCVM were mostly in the Southern US. Most of these counties corresponded to the highest-risk phenotypes G (Impoverished) and F (Middle Class—Inactive), which were mostly distributed across the American South and the Appalachian region, especially in Kentucky, West Virginia, Mississippi, Arkansas, southern Alabama, southern Georgia, southern Missouri, and New Mexico for phenotype G. In contrast, many populous coastal counties in the Northeast and the West were of phenotype A (Affluent—Active), the lowest-risk phenotype. Counties of phenotype B (Affluent—Inactive—Food Secure), the second lowest risk phenotype, were mostly found in the Northeast and the Midwest. A large proportion of counties of phenotype C (Affluent—Inactive—Food Insecure—Excessive Drinking) were found in rural New York and Pennsylvania, as well as in many counties in the Midwest, West, and the state of Texas. Many counties of phenotype D (Middle Class—Active), the median-risk phenotype, were in rural areas of the West, including Arizona, California, Oregon, Washington, and Idaho. Counties of phenotype E (Affluent—Inactive—Food Insecure—No Excessive Drinking) were scattered in a few states in the South and the Midwest, such as Oklahoma, Indiana, and North Carolina.
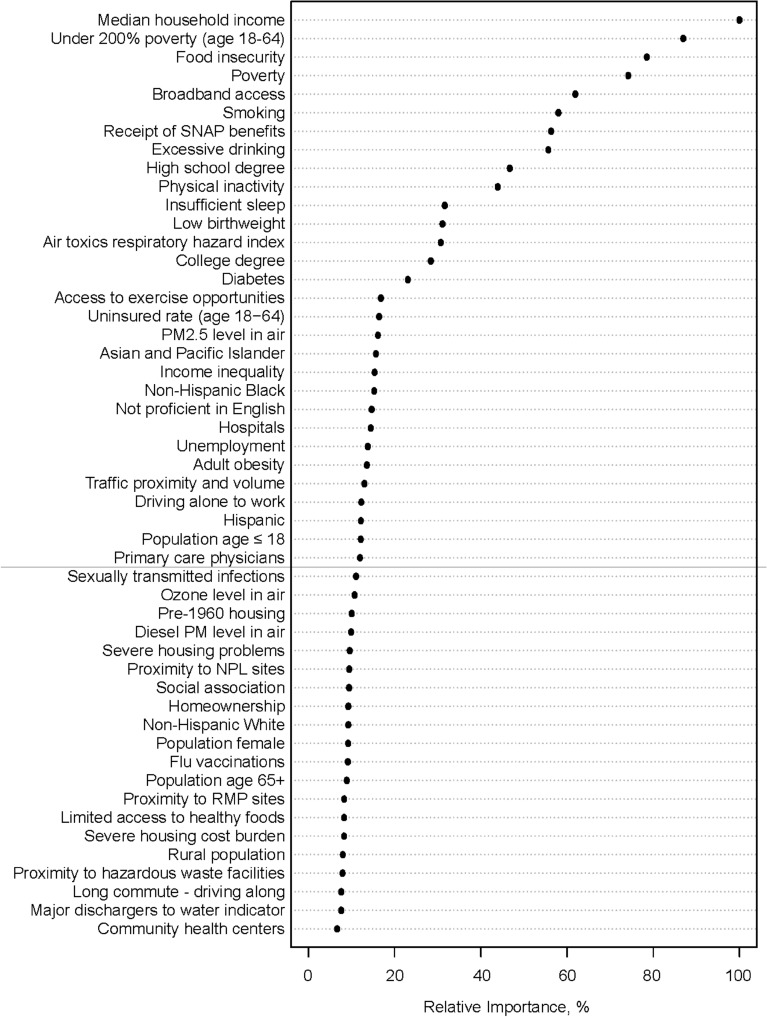
The relative importance of risk factors in predicting PCVM in Fig. 4 suggested that variables that appeared in the CART output were also among the top-ranking variables in the random forest analysis. Notably, median household income, under 200% poverty, and food insecurity were the top three important variables in the random forest plot. Other high-importance variables included broadband access, smoking, and receipt of SNAP benefits, which also appeared in the output of the CART analysis with a minimum number of 100 counties in terminal nodes (Supplementary Fig. S3). Excessive drinking, high school degree, and physical inactivity were ranked 8th to 10th in the variable importance plot.
Figure 4.
Relative importance plot of risk factors in predicting county-level age-adjusted premature cardiovascular mortality from the random forest analysis. Notes: the most important variable is at the top and scaled to 100%. The importance of the rest of the variables is shown relative to the top variable. Abbreviations: SNAP supplemental nutrition assistance program; PM fine particulate matter; RMP risk management plan; NLP national priorities list.
Discussion
Our study identified county phenotypes of PCVM and examined their geographic distributions using machine learning approaches and geographic information systems. We found an approximately threefold difference in the PCVM comparing the highest-risk phenotype in the American South, an area termed the stroke belt due to high rates of stroke16, with the lowest-risk phenotype in the coastal areas in the Northeast and the West.
Our findings suggest that counties of the highest-PCVM-risk phenotype were highly impoverished. The association between poverty and PCVM has been identified by numerous studies1–3. Our study further affirms that income/poverty was the most important predictor of PCVM among various other risk factors related to environmental exposure, health status, health behaviors, and other aspects of socioeconomic status. Previous studies also suggest that physical inactivity was a strong risk factor for PCVM1–3. Our study additionally demonstrated that physical inactivity may be more important in predicting PCVM among counties with higher income than those with lower income (as seen that physical inactivity was a splitting node in the lower poverty group or the higher median household income group in Fig. 1). Similarly, food insecurity, an indicator of dietary behavior and socioeconomic status, may have a stronger association with PCVM among counties with higher physical inactivity (i.e., phenotypes A vs. B). These findings suggest that there may be effect measure modifications between risk factors and their association with PCVM, as may be the case between poverty and physical inactivity, or between physical inactivity and food insecurity.
Notably, counties of the impoverished phenotype (G) and the Middle Class—Inactive phenotype (F), the two highest-risk phenotypes, were mostly located in the American South and the Appalachian region. The concentration of these two phenotypes in the same geographic area provides an opportunity to study in greater detail the interaction between poverty and physical inactivity in the causal pathway to PCVM.
Our study included multiple environmental risk factors in the models. Environmental exposures, especially air pollution, have been mechanistically and epidemiologically linked with disproportionate cardiometabolic outcomes17–19. However, none of the environmental factors appeared in the CART output, nor were they listed as the top ten variables in the random forest plot. On the other hand, multiple studies have demonstrated remarkable overlap between several environmental exposures and socioeconomic factors20, with significant effect interactions between factors such as air pollution and social vulnerability7. One reason behind this discordance is that individuals within counties may have been disproportionately exposed to pollutants, and it is difficult to evaluate to which groups and to what extent of individuals were exposed to the pollutants using data from the current study. Future studies should focus on associations between environmental factors and PCVM at a finer geographic scale.
We also note that risk factors not presented in the CART output may be still highly associated with PCVM, such as broadband access, smoking, receipt of SNAP benefits, and high school education, as suggested by the random forest variable importance plot.
There are several methodological advantages that lend confidence to our study. First, unlike traditional statistical methods (such as regression analysis), CART and random forest machine learning methods can handle a large number of highly correlated variables simultaneously without concerns about multicollinearity due to their variable selection and bootstrap sampling strategies. A second advantage of our methods is that CART has the advantage of visualizing and conceptualizing phenotypes, while random forest complements CART in risk factor importance evaluation and model stability. Specifically, CART selects variables and presents “pathways” for each observation towards its “destination”, where the characteristics along the “pathways” can be used to determine phenotypes associated with PCVM. On the other hand, random forest evaluates all risk factors on their relative importance, including those not selected by CART. Additionally, the variable importance plot of random forest is less sensitive to changes in the data (such as using different years of data) compared to the result of the single-tree CART algorithm.
The above advantages of using CART and random forest methods, together with geographic information systems, have been demonstrated in a prior study investigating the phenotypes of late-stage breast cancer diagnosis21 and cancer mortality22. This study further demonstrates the validity of this approach in uncovering the combination of risk factors and their relative importance in predicting county-level PCVM.
Limitations
The findings of our study should be interpreted within the context of its limitations. First, the accuracy of diagnostic codes from death certificates cannot be ascertained, and there might be additional exposures and proximal contributors to mortality that we were not able to capture. Second, the data collection period for the risk factors did not perfectly match that for the PCVM data, which may be problematic if there is a temporal lag in the effect of risk factors on PCVM. Future studies should explore temporal associations between risk factors and PCVM. Third, data for many risk factors were collected from self-reported surveys based on a sample of the population, where the quality of reporting, response rates, and selection bias may impact the accuracy of the measures. Fourth, to ensure statistical stability, our analyses excluded counties with less than 20 deaths caused by CVD, which might have led to a bias towards less populated areas, especially in the many states in the West and Midwest. Future studies should consider regionalization methods, such as the Max-P-regions model23, to combine counties with small numbers of cases. Finally, counties are relatively large geographic units with seemingly heterogeneous populations and exposures. Whether the associations discovered in the current study are also present in smaller geographic scales (e.g., census tracts or block groups) or at the individual level with long-term cardiovascular outcomes remains to be elucidated. Nevertheless, this proof-of-concept study provides a platform for characterizing the relationships between community-level risk factors and health outcomes.
Conclusion
The use of CART and random forest machine learning methods and geographic information systems can help uncover risk factor associations in predicting PCVM. Interventions to reduce PCVM should be tailored and target geographic areas with high-risk phenotypes of PCVM.
Supplementary Information
Acknowledgements
This study was partly funded by the National Institutes of Health (Awards# P50MD017351, R35ES031702, R01ES019616).
Author contributions
W.D. contributed to the study concept and design, analysis, acquisition, and interpretation of data, and manuscript writing. S.G.A. contributed to the study concept and design, analysis, acquisition, and interpretation of data, manuscript writing, funding acquisition, and supervision. I.M. contributed to analysis, acquisition, and interpretation of data, and manuscript writing. S.R. was involved in the writing and critical revision of the manuscript and funding acquisition. K.N., Z.C, U.K., Y.K., D.F., S.G, and S.R. were involved in the writing and critical revision of the manuscript.
Data availability
The data sets generated during this study are available from the corresponding author upon reasonable request.
Competing interests
Dr. Dong is supported by contracts from Cleveland Clinic Foundation, including a subcontract from Celgene Corporation. Dr. Dong does not have other competing interests to report. Dr. Motairek, Dr. Nasir, Mr. Chen, Dr. Kim, Dr. Khalifa, Dr. Freedman, Dr. Griggs, Dr. Rajagopalan, and Dr. Al-Kindi do not have any competing interest.
Footnotes
The original online version of this Article was revised: The original version of this Article contained an error in the Results section. Full information regarding the corrections made can be found in the correction notice for this Article.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/27/2023
A Correction to this paper has been published: 10.1038/s41598-023-32047-z
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-30188-9.
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics—2022 update: A report from the American Heart Association. Circulation. 2022;145(8):e153–639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.Jin Y, Song S, Zhang L, et al. Disparities in premature cardiac death among US counties from 1999–2017: Temporal trends and key drivers. J. Am. Heart Assoc. 2020;9:e016340. doi: 10.1161/JAHA.120.016340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritchey MD, Wall HK, George MG, Wright JS. US trends in premature heart disease mortality over the past 50 years: Where do we go from here? Trends Cardiovasc. Med. 2020;30:364–374. doi: 10.1016/j.tcm.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth GA, Dwyer-Lindgren L, Bertozzi-Villa A, et al. Trends and patterns of geographic variation in cardiovascular mortality among US counties, 1980–2014. JAMA. 2017;317:1976–1992. doi: 10.1001/jama.2017.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghani AR, Mughal MS, Kumar S, et al. The contemporary trends and geographic variation in premature mortality due to heart failure from 1999 to 2018 in the United States. Int. J. Cardiol. Heart Vasc. 2021;34:100812. doi: 10.1016/j.ijcha.2021.100812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan SU, Javed Z, Lone AN, et al. Social vulnerability and premature cardiovascular mortality among US counties, 2014 to 2018. Circulation. 2021;144:1272–1279. doi: 10.1161/CIRCULATIONAHA.121.054516. [DOI] [PubMed] [Google Scholar]
- 7.Bevan GH, Freedman DA, Lee EK, Rajagopalan S, Al-Kindi SG. Association between ambient air pollution and county-level cardiovascular mortality in the United States by social deprivation index. Am. Heart J. 2021;235:125–131. doi: 10.1016/j.ahj.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Wide-Ranging Online Data for Epidemiologic Research (WONDER). Underlying Cause of Death 1999–2019. Accessed January 20, 2022. https://wonder.cdc.gov.
- 9.University of Wisconsin Population Health Institute. County health rankings & roadmaps. Accessed February 22, 2022. https://www.countyhealthrankings.org.
- 10.Health Resources and Services Administration. Area health resources files. Accessed February 22, 2022. https://data.hrsa.gov/topics/health-workforce/ahrf.
- 11.US Environmental Protection Agency. EJSCREEN: Environmental justice screening and mapping tool. Accessed January 30, 2022. Available at: https://www.epa.gov/ejscreen.
- 12.Lewis, R. J. An introduction to classification and regression tree (CART) analysis, “presented at annual meeting of the society for academic emergency medicine. In Annual Meeting of the Society of Academic Emergency Medicine (2000).
- 13.Breiman L. Random forests. Mach. Learn. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 14.Hothorn T, Hornik K, Zeileis A. Unbiased recursive partitioning: A conditional inference framework. J. Comput. Graph. Stat. 2006;15:651–674. doi: 10.1198/106186006X133933. [DOI] [Google Scholar]
- 15.Ryo M, Rillig MC. Statistically reinforced machine learning for nonlinear patterns and variable interactions. Ecosphere. 2017;8:e01976. doi: 10.1002/ecs2.1976. [DOI] [Google Scholar]
- 16.Lanska DJ, Kuller LH. The geography of stroke mortality in the United States and the concept of a stroke belt. Stroke. 1995;26:1145–1149. doi: 10.1161/01.STR.26.7.1145. [DOI] [PubMed] [Google Scholar]
- 17.Rajagopalan S, Al-Kindi SG, Brook RD. Air pollution and cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2018;72:2054–2070. doi: 10.1016/j.jacc.2018.07.099. [DOI] [PubMed] [Google Scholar]
- 18.Al-Kindi SG, Brook RD, Biswal S, Rajagopalan S. Environmental determinants of cardiovascular disease: Lessons learned from air pollution. Nat. Rev. Cardiol. 2020;17:656–672. doi: 10.1038/s41569-020-0371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph JJ, Deedwania P, Acharya T, et al. Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: A scientific statement from the american heart association. Circulation. 2022;145:e722–e759. doi: 10.1161/CIR.0000000000001040. [DOI] [PubMed] [Google Scholar]
- 20.Jbaily A, Zhou X, Liu J, et al. Air pollution exposure disparities across US population and income groups. Nature. 2022;601:228–233. doi: 10.1038/s41586-021-04190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong W, Bensken WP, Kim U, Rose J, Berger NA, Koroukian SM. Phenotype discovery and geographic disparities of late-stage breast cancer diagnosis across U.S. counties: A machine learning approach. Cancer Epidemiol. Biomark. Prev. 2022;31:66–76. doi: 10.1158/1055-9965.EPI-21-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong W, Bensken WP, Kim U, et al. Variation in and factors associated with US county-level cancer mortality, 2008–2019. JAMA Netw. Open. 2022;5:e2230925–e2230925. doi: 10.1001/jamanetworkopen.2022.30925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duque JC, Anselin L, Rey SJ. THE MAX-P-REGIONS PROBLEM*. J. Reg. Sci. 2012;52:397–419. doi: 10.1111/j.1467-9787.2011.00743.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during this study are available from the corresponding author upon reasonable request.