Abstract
Introduction:
Repetitive transcranial magnetic stimulation (rTMS) is a promising therapeutic technique, and is believed to accomplish its effect by influencing the stimulated and remotely connected areas. However, responsiveness to rTMS shows high interindividual variability, and this intersubject variability is particularly high in older adults. It remains unclear whether baseline resting-state functional connectivity (rsFC) contributes to this variability in older adults. The aims of this study are to (1) examine rTMS effects over the primary motor cortex (M1) in older adults, and (2) identify baseline network properties that may contribute to the interindividual variability.
Methods:
We tested response to intermittent theta burst stimulation (iTBS), an effective rTMS protocol, over M1 by using both electromyography and resting-state functional magnetic resonance imaging in older adults. Outcome measures included motor-evoked potential (MEP) elicited by single-pulse transcranial magnetic stimulation and rsFC before and after an iTBS session.
Results:
iTBS significantly increased MEP amplitudes and rsFC between the stimulation site, sensorimotor cortex, and supplementary motor area (SMA) in older adults. iTBS-induced changes in MEP amplitude were positively correlated with increases in interhemispheric rsFC after iTBS. Furthermore, older adults with lower baseline interhemispheric rsFC between sensorimotor cortex and SMA exhibited stronger MEP response after iTBS.
Discussion:
Findings of the study suggest that different levels of interhemispheric communication during resting state might contribute to the response heterogeneity to iTBS in older adults. Interhemispheric rsFC may have great potential serving as a useful marker for predicting iTBS responsiveness in older adults. ClinicalTrials.gov ID: 1707654427
Impact statement
Factors contributing to interindividual variability of the responsive to repetitive transcranial magnetic stimulation (rTMS) in older adults remain poorly understood. In this study, we examined the effects of rTMS over the primary motor cortex in older adults, and found that response to rTMS is associated with prestimulation interhemispheric connectivity in the sensorimotor and premotor areas. Findings of the study have great potential to be translated into a connectivity-based strategy for identification of responders for rTMS in older adults.
Keywords: functional connectivity, interindividual variability, older adults, theta burst stimulation, transcranial magnetic stimulation (TMS)
Introduction
Transcranial magnetic stimulation (TMS) is a noninvasive brain stimulation technique that has been used to measure neurophysiological properties of the human motor cortex and descending corticospinal system in vivo. TMS works by discharging a short but powerful electric current through a wire coil that is carefully placed on the surface of the scalp, and this produces a rapidly alternating magnetic field. The magnetic field strength is no stronger than what is used in standard magnetic resonance imaging (MRI; 1–2 Tesla), but as explained by Faraday's Law, this magnetic flux can induce a secondary electric current in superficial cortical tissue (Ueno et al., 1988). With the sufficient outward current, TMS pulses can directly depolarize neurons that are at a depth of 2–3 cm underneath the skull (Deng et al., 2013), and the resultant action potentials will propagate to distal regions through functionally and/or structurally connected circuitry (Chou et al., 2015).
TMS is a versatile tool, which has been used to investigate neurophysiology of the motor system, quantify integrity of brain circuitries, and induce changes in cortical excitability. Single-pulse TMS, for example, can be applied to the primary motor cortex (M1), whereby action potentials propagate synchronously down the corticospinal tract to elicit a motor response in the peripheral muscles that correspond with the stimulated brain region. This synchronized myogenic response can be measured with electromyography (EMG) and is referred to as the motor-evoked potential (MEP). Beyond single-pulse TMS, technical advances have enabled the delivery of so-called repetitive TMS (rTMS), whereby stimuli are discharged in repetitive trains at specific frequencies.
Distinct from single-pulse TMS, rTMS is known to modulate cortical excitability with its effect lasting up to 60 min (Gersner et al., 2011; Wischnewski and Schutter, 2015). Moreover, TMS can be leveraged to probe neural plasticity (or changes in cortical excitability) in M1 by interleaving single-pulse TMS-induced MEP measures with an rTMS paradigm, whereby the rTMS-induced change in MEPs is indicative of transient neural reorganization that resembles long-term potentiation (LTP) or long-term depression (LTD).
In this study, we aim to investigate the transient neural reorganization in response to rTMS and explore individual variability in older adults. The transient neural reorganization in older adults would be measured using both EMG and resting-state functional magnetic resonance imaging (rs-fMRI). Although previous studies in younger adults have reported that facilitatory rTMS protocols (i.e., high frequency, intermittent theta burst stimulation [iTBS], etc.) applied over M1 tend to enhance cortical excitability (Hoogendam et al., 2010; Pell et al., 2011), and that the enhancement of cortical excitability is associated with a stronger coupling of resting-state functional connectivity (rsFC) between the stimulation site and remote brain regions (Chou et al., 2015; Lee et al., 2018; Min et al., 2016; Moisa et al., 2009; Nettekoven et al., 2015; Tik et al., 2017), there is considerable heterogeneity in responses to rTMS (Guerra et al., 2020; Hinder et al., 2014).
For example, in the study conducted by Hamada et al. (2013), only 25% of healthy younger participants demonstrated the expected response in cortical excitability to both facilitatory and inhibitory rTMS. A few studies have explored potential factors (e.g., brain state, genetics, age, circadian cycle, or TMS parameters) that may modulate responsiveness to rTMS (Hamada et al., 2013; Tecchio et al., 2008; Todd et al., 2010). One potentially important but underinvestigated factor is the prestimulation brain state with respect to large-scale brain networks that can be measured by rs-fMRI. This functional architecture of the brain can be characterized as a dynamic interactive network system by examining temporal correlations in the blood oxygen level-dependent (BOLD) signals of interconnected brain regions with rs-fMRI. These temporal correlations enable both the investigation of rTMS effects on a network level, and how prestimulation network characteristics may moderate responsiveness to subsequent rTMS.
Previous studies in healthy younger adults reported that participants with lower levels of baseline fMRI BOLD signals showed stronger facilitation effects after facilitatory rTMS (Cárdenas-Morales et al., 2014). In another study in younger adults (Hordacre et al., 2017), weaker M1-frontocentral network connectivity was associated with greater response to inhibitory rTMS. The association between prestimulation network connectivity and rTMS-induced therapeutic effects has also been reported in depression (Salomons et al., 2014). While these findings are relatively consistent, the heterogeneity of rTMS response has been mostly studied in healthy younger adults and patients with depression, with scarce investigation into this variability among older adults.
Here, we employ a special type of rTMS protocol, iTBS (Huang et al., 2005), to investigate interindividual variability in older adults by examining the contribution of baseline rsFC to the iTBS effects. Examining the degree to which the rsFC modulates iTBS effects is particularly relevant in older adults because age-related reorganization of functional brain networks has been indicated by many studies (Barkhof et al., 2014; Sala-Llonch et al., 2015), and this age-related reorganization or adaptation may lead to individual variability in network functioning and response to brain stimulation (Goh, 2011; Jockwitz and Caspers, 2021; Tang et al., 2019).
There is considerable evidence showing that aging is associated with a number of physiological modifications, including impairment in dendritic morphology and loss of synaptic cellular connectivity (Godde et al., 2002; Tang et al., 2019). Aging is also related to a decrease in excitability of inhibitory circuits within M1, which can result in alterations in interhemispheric inhibition (Peinemann et al., 2001). Although age-related increase in recruitment of brain regions has been reported in older adults (Cabeza, 2002), the role this increased recruitment plays in brain function remains an object of debate.
The increased activation could be either beneficial as a compensatory mechanism or detrimental (Bernard and Seidler, 2012; Crosson et al., 2015; Langan et al., 2010; Madden et al., 1999; McGregor et al., 2011). The aim of the study is to investigate the impact of baseline brain state as measured by rsFC on the iTBS effects in older adults. Given that previous studies have found that baseline rsFC of motor network was associated with response to rTMS in younger adults (Minkova et al., 2019; Nettekoven et al., 2015; Volz et al., 2013), we hypothesize that rsFC of motor network before iTBS would moderate neuroplastic responses to iTBS in older adults.
Methods
Participants
Nineteen right-handed healthy older adults (11 women and 8 men) aged 63–74 (69.4 ± 3.1) years participated in our study. Individuals with contraindications to TMS or MRI were excluded. No participants enrolled in this study reported taking medications for neurological or psychiatric diseases. The research protocol was approved by the University Institutional Review Board (IRB), and each participant provided written informed consent before their participation. Data from three participants were excluded from the final analysis because their MEP data were identified as outliers (see Results section).
Experimental design
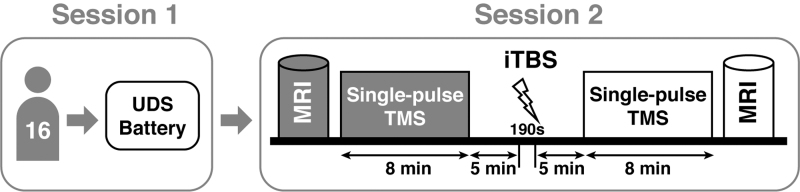
Each participant attended two experimental sessions, each taking ∼3 h to complete. In the first session, we measured cognitive function using National Alzheimer's Coordinating Center Uniform Data Set (UDS) Neuropsychological Battery Version 3 for each participant. This battery assessed different cognitive domains, including episodic memory, processing speed, executive function, language, constructional ability, and visuoconstructional skills (Weintraub et al., 2018). In the second session, participants underwent both MRI and TMS. First, pre-iTBS MRI data were acquired, including structural T1 MRI used to precisely localize the stimulation site for each participant and resting-state fMRI for estimation of baseline functional connectivity.
Second, single-pulse TMS was applied to the left primary motor cortex (M1) hotspot for right abductor pollicis brevis (APB) thumb muscle to assess the baseline cortical excitability. Third, after 5–10 min break, iTBS was applied to the motor hotspot to modulate cortical excitability. Fourth, we obtained post-iTBS single-pulse TMS outcome measures. Finally, post-iTBS resting-state fMRI data were acquired. Figure 1 shows the overview of the experimental procedure. Specific methods are described in the following subsections.
FIG. 1.
Experimental procedures. Each participant took part in two sessions to complete this study. During the first session, participants underwent the UDS Neuropsychological Battery. During the second session, outcome measures including cortical excitability and resting-state functional connectivity were acquired immediately before and after the iTBS. Single-pulse TMS was applied over the left primary motor cortex to assess aMT and other cortical excitability measures. aMT, active motor threshold; iTBS, intermittent theta burst stimulation; MRI, magnetic resonance imaging; TMS, transcranial magnetic stimulation; UDS, Uniform Data Set.
TMS protocols
TMS was delivered through a MagPro x100 system (MagVenture Ltd.) with a CB60 figure-of-eight coil for single-pulse TMS and a Cool-B65 A/P coil for iTBS. TMS coils, landmarks on the participant's head, and structural T1 MRI scan were coregistered using an infrared-based frameless three-dimensional (3D) neuronavigation system for brain reconstruction (Localite). In the 3D brain model, an anatomical “hand knob” within the left M1 was identified visually. Single-pulse TMS was performed around the “hand knob” to determine the optimal stimulation site (i.e., hotspot) for each participant based on the MEPs of the right APB thumb muscle. The hotspot and coil orientation were recorded for subsequent single-pulse TMS and iTBS sessions.
Single-pulse TMS
The single-TMS session consisted of measuring active motor threshold (aMT) and active input–output (aIO) curve. Single biphasic pulse TMS was delivered to the previously determined “hotspot” located in the left M1 to generate MEPs of the right APB thumb muscle using surface EMG. EMG signals were acquired through Spike 2.0 software and analog-to-digital converter. These signals were then amplified by 5000-folds with a bandpass filter between 30 and 1000 Hz and a line filter (60 Hz notch). aMT was measured while participants were instructed to gently activate the right APB thumb muscle.
To sustain a consistent tonic activation level throughout the measurement, participants were instructed to match their real-time EMG signal to a target level corresponding to 20% of their maximum muscular activation through visual feedback displayed on a monitor. aMT refers to the minimal TMS intensity that is required to elicit motor output and was measured using a freeware program that employs maximum-likelihood parameter estimation by sequential testing strategy without the need for a priori information (TMS Motor Threshold Assessment Tool 2.0). The same tonic activation level was maintained during the subsequent aIO curve procedure. aIO curve describes how MEP amplitude changes with stimulus intensity.
MEP amplitudes were obtained by postprocessing EMG signals using MATLAB to calculate peak-to-peak MEP amplitude values between 20 and 50 msec after the onset of each TMS pulse. Sixteen different stimulus intensity levels relative to aMT were delivered using preprogrammed sequence. Sixteen stimulus levels were 80%, 90%, 95%, …, 150%, 155%, 165% of aMT with four repeats at each stimulus level, yielding a total of 64 trials. The order of stimuli was pseudorandomly shuffled with intertrial intervals that were also pseudorandomly varied between 6 and 9 sec with 1 sec resolution. This method could minimize potential sequence effect that was observed in earlier studies (Rossini et al., 2015).
Intermittent theta burst stimulation
The iTBS protocol comprised of 600 biphasic pulses at 80% of aMT. The stimuli were patterned in 3-pulse bursts at 50 Hz, which repeated at a frequency of 5 Hz, and were delivered in intermittent trains, each train lasting 2 sec with a 6.9 sec intertrain interval (Huang et al., 2005). The TMS coil was positioned at the same motor hotspot identified for the single-pulse TMS using the 3D neuronavigation system.
MRI acquisition
All brain images were acquired using MAGNETOM® Skyra 3 Tesla MRI scanner (Siemens Medical Systems, Erlangen, Germany) with a 32-channel receiver head coil. Foam pads were applied to prevent head motion. The localizer was collected using the following parameters—direction: inferior to superior, repetition time (TR) = 8.6 msec, echo time (TE) = 4.0 msec, flip angle = 20, field-of-view (FOV) = 250 mm, 5 slices, 7 mm slice thickness.
High-resolution structural images were acquired using a T1-weighted spoiled gradient recalled sequence (TR = 2530 msec; TE = 3.3 msec; FOV = 25.6cm; flip angle = 20°; in-plane matrix size = 256 × 256; slice thickness = 1 mm; number of slices = 176). Whole-brain resting-state fMRI data were collected using T2*-weighted echo-planar imaging pulse sequence: FOV = 240 mm; TR = 3000 msec; TE = 36 msec; flip angle = 90°; in-plane acquisition matrix size = 160 × 160; voxel size = 1.5 mm3; and multiband factor = 2; scan time = 8 min), resulting in functional data from 39 axial slices with isotropic voxels of 4 mm3. During the resting-state fMRI scan, participants fixated their eyes on a crosshair with eyes open.
Outcome measures and statistical analysis
The outcome measures included cortical excitability and rsFC. See below.
Cortical excitability
Both amplitude of MEPs and active input–output curve (aIO curve) parameters in response to single-pulse TMS would be used to measure cortical excitability. We used principal component analysis (PCA) to extract a dominant component among changes in MEPs (ΔMEPs change = Post-iTBS − Pre-iTBS) across 13 TMS intensities (i.e., 100–165% of aMT). PCA is a mathematical procedure that reduces the dimensionality of a data set consisting of many intercorrelated variables while retaining the variance in the data set as much as possible. In addition to the amplitude of MEPs, the first component score (λ1ΔMEPs) generated by the PCA for each individual was used for statistical analysis.
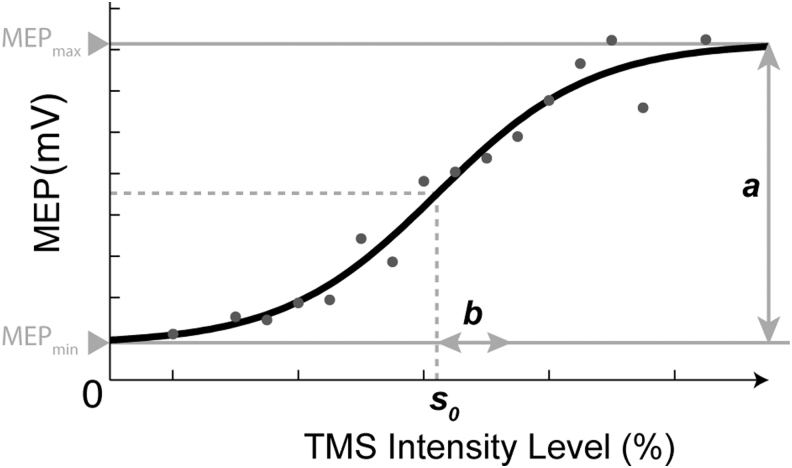
For the aIO curve parameters, the amplitudes of MEP (AMEP) were used to model the aIO curve as a function of TMS intensity (s) by fitting the data to a Boltzmann equation (Devanne et al., 1997). The Boltzmann equation [Eq. (1)] has three free parameters: a, b, and s0. As shown in Figure 2, a corresponds to the change in MEP amplitude across stimulation intensities (i.e., maximum MEP amplitude minus minimum MEP amplitude), b corresponds to the spread of the curve, and s0 is the sensitivity (i.e., midpoint of the curve = TMS intensity at which 0.5a + ɛ0 was elicited). The fitting was performed for each individual participant, and for each aIO curve acquired immediately before and after iTBS. The fitting parameters were constrained conditionally to ensure convergence. The bottom plateau in aIO curve is the active muscle contraction level (ɛ0), hence constrained by taking the prestimulus peak-to-peak between −100 and 0 msec.
FIG. 2.
Parameters derived from aIO curve fitted by the Boltzmann equation. a = Change in amplitude of MEP across TMS intensities, b = the spread of the curve, s0 = sensitivity index (i.e., midpoint of the curve = TMS intensity at which 0.5a + ɛ0 was elicited). aIO, active input–output; MEP, motor-evoked potential.
| (1) |
Participants from our study were self-reported healthy older adults. The UDS Neuropsychological Battery was used to evaluate participants' cognitive function. As aforementioned, the UDS Neuropsychological Test Battery provides a comprehensive assessment of cognitive status, and has been extensively used in cognitively normal older adults, individuals with subjective cognitive complaints, and patients with mild cognitive impairment. This assessment focused on domains, including attention, processing speed, executive function, episodic memory, and language.
Tests included the Montreal Cognitive Assessment, Digit Span (Forward and Backward), Trail Making Test (Part A and Part B), Benson Complex Figure (Immediate and Delayed), Category Fluency-Animals, Category Fluency-Vegetables, and symbol search task (Weintraub et al., 2018). Although our participants are self-reported healthy older adults, variabilities existed in the neuropsychological data across participants (Supplementary Table S1). Therefore, we used the neuropsychological data as a covariate in our analyses to control for the potential confounding factor of cognitive disparities. The PCA was applied to the 16 neuropsychological variables derived from the UDS Neuropsychological Battery, and the first principal component that accounted for most of the variance would be included as a covariate in the analysis.
We compared the mean changes in MEPs among 13 levels of TMS intensities (i.e., 100–165% aMT) between pre-iTBS and post-iTBS (TIME) with a two-way repeated-measures analysis of covariance (ANCOVA; INTENSITY × TIME), including the UDS Neuropsychological Battery score as a covariate. Data normality was assessed using the Kolmogorov–Smirnov and Shapiro–Wilk test. The homogeneity of variances was tested using Levene's test. Cortical excitability was also assessed by the λ1,ΔMEPs derived from PCA and aIO curve parameters, including a, b, and s0. The percentage changes in aIO curve parameters were computed as a ratio of post-iTBS value divided by pre-iTBS value (e.g., Δa% = Post-iTBS a/Pre-iTBS a) for each participant. One-sample t-test was performed on λ1,ΔMEPs, Δa%, Δb%, and Δs0% to examine the iTBS effect on cortical excitability.
Resting-state functional connectivity
Resting-state fMRI data were preprocessed with realignment, unwarping (subject motion estimation and motion correction), slice timing correction, outlier detection (composite movement from preceding image >0.5 mm), simultaneous gray/white/cerebrospinal fluid segmentation and MNI normalization and smoothing with Gaussian kernel at 8 mm full-width-at-half-maximum. Preprocessing procedure was performed with the Statistical Parametric Software (SPM; Wellcome Department of Imaging Science, Functional Imaging Laboratory, University College London) and CONN functional connectivity toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012).
We first conducted the seed-to-voxel analysis by estimating how iTBS changed the voxel-wise functional connectivity in the whole brain using each individual's motor hotspot as a seed. Each participant's hotspot (i.e., stimulation site) was recorded using the LOCALITE system (Localite Version 3.0.41; Polaris System), and the Talairach coordinate was exported for analysis. The Talairach coordinate was then converted into MNI coordinate. We created a spherical seed with a radius of 5 mm centered at the individual MNI coordinate (averaged MNI across participants: [−36, −20, 48]) by fslmaths (Jenkinson et al., 2012). At the individual-level analysis, the design matrix included two sessions (Pre-iTBS and Post-iTBS) plus six motion parameters and their temporal derivatives derived from outlier identification, segmented white matter, and cerebrospinal fluid mean signal as confounding variables.
All images were bandpass filtered (0.008–0.09 Hz). The fMRI signal time course was averaged across all voxels within the seed region. We computed the correlation coefficients between the averaged time course of the seed and the time course of each voxel in the whole brain for all participants. The z-transformed Pearson correlation maps were brought to the second-level group analysis. Contrasts from the first level individual analysis was fed into second-level seed-to-voxel analysis. The event time of pre-iTBS and post-iTBS were coded into the general linear model. To account for the variance in cognitive performance present in our sample of older adults, we added the UDS Neuropsychological Battery score as a covariate. Voxel-wise analysis result was presented at a p < 0.05 false discovery rate-corrected cluster-wise threshold, with the minimum cluster size = 20 voxels and a p < 0.001 uncorrected voxel-wise threshold.
We next performed the region-of-interest (ROI) analysis to estimate the changes in interhemispheric rsFC and baseline interhemispheric rsFC. The whole-brain functional connectivity matrix was calculated across the time series using the Fisher z-transform correlation coefficient map among 69 ROIs defined by the automated anatomical labeling atlas (Tzourio-Mazoyer et al., 2002). We obtained the correlation coefficient maps (69 × 69 regions) for rsFC before and after iTBS.
Correlations between outcome measures
The relationship between λ1ΔMEPs derived from PCA and changes in rsFC after iTBS was assessed using Pearson's partial correlations including the UDS Neuropsychological Battery score as a covariate. A partial correlation was also conducted to assess the relationship between the baseline rsFC and λ1ΔMEPs. In addition, we assessed correlations between λ1ΔMEPs and aMT, a, b, and s0. Pearson's partial correlations were computed by including the UDS Neuropsychological Battery score as a covariate. The representativeness of the PCA-derived first principal component score (λ1ΔMEPs) for the averaged changes in MEP amplitude after iTBS was examined with Pearson's correlation. A p-value of <0.05 (two tailed) was considered statistically significant.
Results
iTBS significantly increased MEP amplitudes
We used two-way repeated-measures ANCOVA to test TMS intensity (13 levels: 100–165% of aMT), iTBS (2 levels: pre-iTBS and post-iTBS), and their interaction effects on MEP amplitudes while controlling for UDS Neuropsychological Battery score. MEPs data at all TMS intensity levels are normality distributed, as indicated by p-values >0.05 followed by the Kolmogorov–Smirnov and Shapiro–Wilk tests of normality. Levene's test was not significant, suggesting homogeneity of the residual variance across all participants. The two-way repeated-measures ANCOVA revealed a significant main effect of intensity, F(1, 512) = 209.54, p < 0.001, and a significant INTENSITY × TIME interaction effect, F(1, 512) = 120.01, p = 0.0403.
Post hoc analyses of the interaction effect using pairwise comparisons with Bonferroni corrections showed that significant increases in MEP amplitudes after iTBS were observed at the single-pulse TMS intensities at 110% aMT (p = 0.016) and 165% aMT (p = 0.015) (Table 1 and Fig. 3). We used PCA to extract a dominant component among changes in amplitudes of MEPs (ΔMEPs change = Post-iTBS − Pre-iTBS) across the 13 suprathreshold TMS intensity levels (i.e., 100–165% of aMT). The first principal component score of changes in MEP amplitudes after iTBS accounted for 37.9% of the total variance. Instead of using 13 different TMS intensities, the score of the first principal component with the most variance explained was used to represent changes in MEP amplitude after iTBS. The first principal component was dominated by positive loadings >0.30 for MEP changes in response to iTBS at 110%, 105%, 155%, 120%, 145%, and 115% aMT (Supplementary Table S2). One-sample t-test on λ1ΔMEPs was significantly >0, t(15) = 2.593, p = 0.01.
Table 1.
Summary of Intermittent Theta Burst Stimulation Effects on Cortical Excitability Measures
| Outcome measures | Baseline (PreiTBS) | Difference (PostiTBS − PreiTBS) | t-Score | p |
|---|---|---|---|---|
| MEPs at 110% aMT | 549 ± 380 | 102 ± 178 | 2.489 | 0.016 |
| MEPs at 165% aMT | 1329 ± 547 | 183 ± 338 | 2.360 | 0.015 |
| a (μV) | 1417 ± 837 | 381 ± 845 | 1.893 | 0.039 |
| s0 (% MSO) | 60.8 ± 14.5 | 1.2 ± 5.9 | 0.825 | 0.211 |
| b (% MSO) | 4.2 ± 1.7 | 0.8 ± 2.0 | 1.638 | 0.061 |
| λ1,ΔMEP | — | 194 ± 299 | 2.593 | 0.010 |
aMT, active motor threshold; iTBS, intermittent theta burst stimulation; MEPs, motor-evoked potentials; MSO, maximum stimulator output.
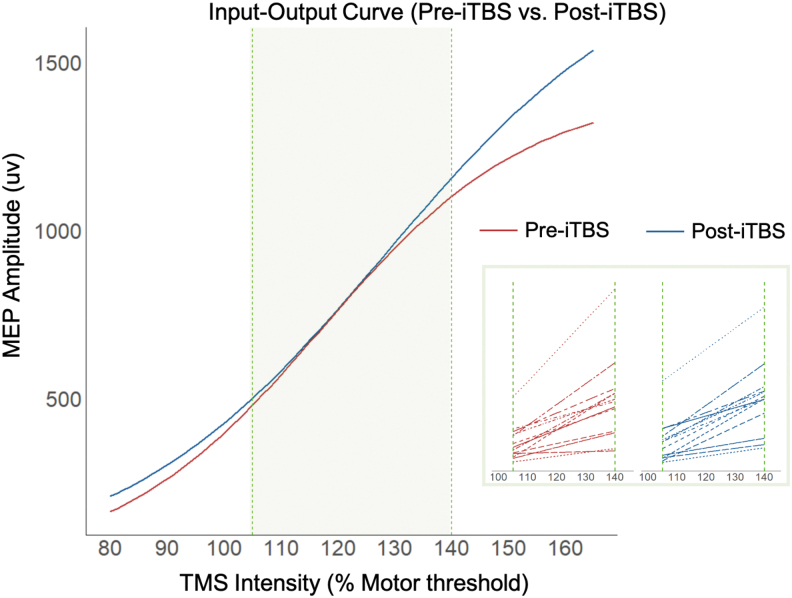
FIG. 3.
The aIO curve was used to model the MEP amplitudes as a function of TMS intensity (% motor threshold) by fitting the data to a Boltzmann equation. Red line denotes the pre-iTBS cortical excitability profile, and blue line denotes the post-iTBS cortical excitability profile. The inset represents the Boltzmann curve fit to the aIO curve of each participant within the green zone for both pre-iTBS and post-iTBS conditions.
The change in MEPs due to iTBS was strongly correlated with λ1ΔMEPs (r = 0.760, F = 21.4, p = 0.0004), indicating that greater λ1ΔMEPs were associated with greater changes in MEP amplitude in response to iTBS. The λ1ΔMEP will be used to correlate with brain functional connectivity data in the following analysis. For the aIO curve parameters (i.e., a, b, and s0), one-sample t-tests revealed that the maximum MEP amplitude (i.e., a in Table 1) increased significantly after iTBS (t = 1.7411, p = 0.039), but the sensitivity measure (s0 in Table 1) and the spread (b in Table 1) of the aIO curve did not show a significant iTBS effect.
iTBS significantly increased rsFC
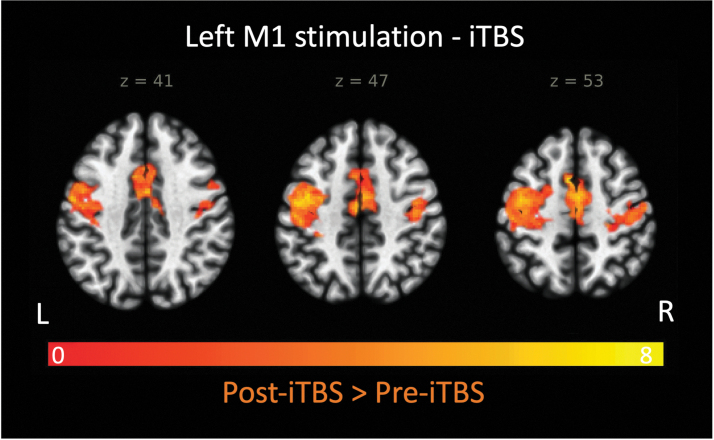
The seed-to-voxel analysis with individual stimulation site as a seed (i.e., hotspot within the left hemisphere responsible for the right thumb movement) yielded significant iTBS effects on the following brain regions: bilateral precentral gyri (M1), bilateral supplementary motor area (SMA), and right postcentral gyrus (S1; p < 0.001, family-wise error corrected at the cluster level, Table 2 and Figure 4). The result indicates that iTBS significantly increased rsFC between the stimulation site and bilateral primary motor, bilateral premotor, and right primary sensory areas.
Table 2.
Brain Regions That Exhibited Increased Resting-State Functional Connectivity with the Stimulation Site after Intermittent Theta Burst Stimulation
| MNI coordinate |
Side | Cluster distribution | Peak t-value | Cluster level |
||||
|---|---|---|---|---|---|---|---|---|
| x | Y | z | Size | P FDR-corr | P uncorr | |||
| −16 | −18 | 62 | L | Precentral gyrus (M1) | 5.58 | 540 | <0.000094 | <0.000001 |
| 12 | −20 | 60 | R | Precentral gyrus (M1) | 4.88 | |||
| −2 | −16 | 66 | L | SMA | 4.16 | |||
| 4 | −10 | 72 | R | SMA | 4.42 | |||
| −30 | −18 | 74 | R | Postcentral gyrus (S1) | 4.37 | |||
FDR, false discovery rate; SMA, supplementary motor area.
FIG. 4.
Brain regions that exhibited increased resting-state functional connectivity with the stimulation site (i.e., left primary motor cortex or M1) after iTBS. Results are cluster-size, FDR corrected for multiple comparisons (Z > 2.92; p < 0.005) and are overlaid on the MNI template. FDR, false discovery rate.
Changes in MEP amplitudes after iTBS were associated with interhemispheric rsFC of sensory motor cortex
Based on the findings that M1, SMA, and S1 exhibited changes in rsFC after iTBS, we used rsFC among six ROIs (i.e., left M1 [MNI: −39, −6, 51], right M1 [MNI: 41, −8, 52], left S1 [MNI: −42, −23, 49], right S1 [MNI: 41, −25, 53], left SMA [MNI: −5, −5, 61], and right SMA [MNI: 9, 0, 62]) to examine relationship between rsFC and changes in MEP amplitudes.
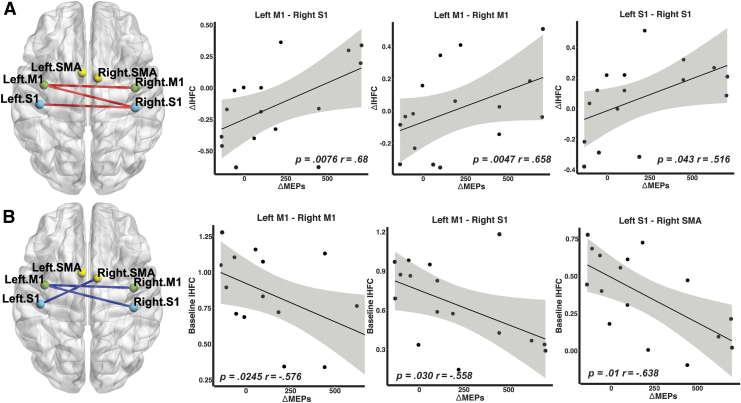
First, we used Pearson's partial correlations to examine the relationship between changes in MEP amplitudes and rsFC after iTBS with UDS Neuropsychological Battery score as a covariate. The iTBS-induced change in MEP amplitudes (i.e., λ1ΔMEPs) was positively correlated with changes in interhemispheric rsFC between (1) left M1 and right M1 (r = 0.658, p = 0.0076), (2) left M1 and right S1 (r = 0.687, p = 0.0047), and (3) left S1 and right S1 (r = 0.516, p = 0.043). In other words, a greater increase in MEP amplitudes was significantly associated with higher increases in interhemispheric rsFC among these ROIs (Fig. 5A).
FIG. 5.
(A) Partial correlations between changes in IHFC (ΔIHFC) and λ1ΔMEPs. Resting-state functional connectivity between the left primary motor cortex (M1) and right somatosensory cortex (S1), between left M1 and right M1, and between left S1 and right S1 was positively correlated with changes in MEPs (i.e., λ1ΔMEPs). The Pearson correlation coefficients (r) and p-values are displayed. The Pearson partial correlations are computed controlling for cognitive performance measured by the UDS Neuropsychological Battery. (B) Partial correlations between baseline IHFC and λ1ΔMEPs in response to iTBS. Resting-state functional connectivity between the left M1 and right M1, between left M1 and right S1, and between left S1 and right SMA was negatively correlated with λ1ΔMEPs. The shaded error bands indicate 95% confidence intervals. IHFC, interhemispheric functional connectivity; SMA, supplementary motor area.
Second, we examined whether baseline rsFC could be used to account for cortical excitability response to iTBS. The change in MEP amplitudes (i.e., λ1ΔMEP) was significantly correlated with the baseline interhemispheric rsFC between (1) bilateral M1 (r = −0.576, p = 0.0245), (2) left M1 and right S1 (r = −0.558, p = 0.030), and (3) left S1 and right SMA (r = −0.638, p = 0.01). Individual with lower baseline interhemispheric rsFC within sensory motor cortex exhibited a greater change in MEP amplitudes after iTBS (Fig. 5B).
aMT and sensitivity were associated with interhemispheric rsFC of sensory motor cortex
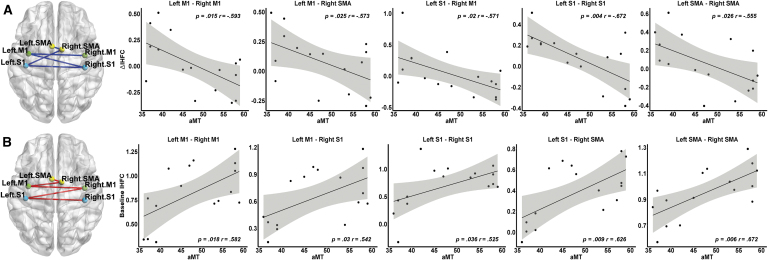
Using the six ROIs (i.e., bilateral M1, S1, and SMA), we found significant negative correlations between aMT and the rsFC changes after iTBS in these ROI pairs: left M1-right M1 (r = −0.5929, p = 0.015), left M1-right SMA (r = −0.5734, p = 0.025), and left S1-right M1 (r = −0.5708, p = 0.02), left S1-right S1 (r = −0.6720, p = 0.004), and left SMA-right SMA (r = 0.555, p = 0.026), indicating that lower aMT was associated with greater increase in interhemispheric rsFC after iTBS (Fig. 6A).
FIG. 6.
(A) Partial correlations between changes in IHFC (ΔIHFC) and aMT. Functional connectivity within the sensory motor cortex was negatively correlated with aMT. (B) Partial correlations between baseline IHFC and aMT are provided. Functional connectivity within the sensory motor cortex was positively correlated with aMT. The Pearson correlation coefficients (r) and p-values are displayed. The Pearson partial correlations are computed controlling for cognitive performance measured by the UDS Neuropsychological Battery. The shaded error bands indicate 95% confidence intervals.
Furthermore, significant positive correlations were found between aMT and baseline interhemispheric rsFC in these ROI pairs: left M1-right M1 (r = 0.5820, p = 0.018), left M1-right S1 (r = 0.5421, p = 0.030), left S1-right S1 (r = 0.5252, p = 0.036), left S1-right SMA (r = 0.626, p = 0.009), and left SMA-right SMA (r = 0.672, p = 0.006), indicating that individuals with lower aMT featured a lower pre-iTBS interhemispheric rsFC (Fig. 6B). However, the relationship between MEP changes and aMT was not statistically significant (r = 0.238, p = 0.098).
Similarly, we found significant negative correlations between s0 (i.e., a sensitivity measure derived from the aIO curve with lower s0 indicating higher sensitivity) and the rsFC changes after iTBS. Significant correlations were found in the following ROI pairs: left M1-right M1 (r = −0.6374, p = 0.0079), left M1-right S1 (r = −0.507, p = 0.0447), and left S1-right M1 (r = −0.5667, p = 0.0221), and left S1-right S1 (r = −0.5479, p = 0.028), showing that lower s0 (higher sensitivity to single-pulse TMS) was associated with greater increase in interhemispheric rsFC after iTBS.
Furthermore, significant positive correlations were found between s0 and baseline interhemispheric rsFC in these ROI pairs: left M1-right M1 (r = 0.6526, p = 0.006), left M1-right S1 (r = 0.7486, p = 0.0008), left S1-right S1 (r = 0.5673, p = 0.0219), left S1-right SMA (r = 0.6312, p = 0.0087), and left SMA-right SMA (r = 0.5927, p = 0.0155), indicating that individuals with lower s0 (higher sensitivity to single-pulse TMS) exhibited a lower pre-iTBS interhemispheric rsFC. s0 was significantly correlated with aMT (r = 0.8575, p < 0.0001), but the relationship between MEP changes and s0 was not statistically significant (r = 0.31, p = 0.0735).
Finally, to examine signs of muscle fatigue during aIO curve measurements, we calculated the mean power spectral density of the EMG signal from the first five trials and the last five trials of the aIO curve measurements. The power spectrum density was extracted from the median power frequency using the Fast Fourier transformation. As suggested from the previous literature (Fuglevand et al., 1993; Kallenberg et al., 2007), a significant decrease in spectral density would indicate muscle fatigue. The paired t-test analysis did not show a significant decrease in power spectral density between the first five trials and the last five trials, t(14) = 1.7769, p = 0.10, indicating that no apparent sign of fatigue was observed during aIO curve measurements.
Discussion
In this study, we investigated excitatory iTBS effects on both the amplitudes of MEP and brain rsFC in older adults. We also explored response heterogeneity to iTBS in older adults. First, iTBS significantly increased MEP amplitudes and rsFC between the stimulation site (i.e., M1) and primary sensory motor cortex and SMA. The increase in MEP amplitudes was significantly associated with positive changes in rsFC after iTBS. Second, baseline interhemispheric rsFC within the sensory motor cortex was significantly correlated with the enhancement of MEP amplitudes.
Association between iTBS-induced changes in MEP amplitudes and functional connectivity
Consistent with previous TMS literature in healthy younger adults (Nettekoven et al., 2015; Volz et al., 2013), we reported positive correlations between changes in MEP amplitudes and rsFC among sensory motor cortex and SMA in older adults (Fig. 5). For example, a study showed that changes in MEP amplitudes after quadri-pulse rTMS were correlated with changes in rsFC between the stimulated M1 and contralateral M1 in younger adults (Watanabe et al., 2013). Similarly, healthy younger adults who exhibited a stronger bilateral motor connectivity among M1 and premotor cortex after single-pulse TMS showed a shorter MEP latency (i.e., the time between single-pulse TMS and onset of MEPs) (Volz et al., 2015).
Previous studies have also shown that TMS over M1 induced significant activation in somatosensory cortex (Denslow et al., 2005; Jung et al., 2020). The local electromagnetic stimulation and TMS coil motion could lead to tapping sensation against the scalp. As a result, activation in the somatosensory area due to scalp stimulation would be expected, and has been reported to occur slightly lateral and inferior to the hand area of the precentral gyrus (Roland et al., 1998). In addition, M1 may play a role in coordinating with somatosensory area for somatic sensation (Naito et al., 2002). Therefore, increased rsFC between M1 and somatosensory cortex after iTBS observed in our study could be attributable to synchronization of signals from M1, afferent/reafferent sensory feedback, and tactile sensation over the scalp.
In addition, the correlations between changes in rsFC and behavior have been reported in clinical populations. For example, successful rTMS treatment for major depression measured by the Hamilton Depression Rating Scale was associated with increase in frontal-striatal-thalamic rsFC (Salomons et al., 2014). Similarly, for rTMS treatment in individuals with obsessive-compulsive disorder, changes in cortical-striatal rsFC after stimulation of the dorsomedial prefrontal cortex correspond with a larger reduction of symptoms (Beuzon et al., 2017; Dunlop et al., 2016; Figee et al., 2013).
In addition, Ackerley et al. used iTBS targeting the ipsilesional M1 to examine treatment efficacy in stroke patients. An asymmetric index was used to indicate the interhemispheric balance of corticomotor functional connectivity. They showed that better motor response was associated with greater improvement in the symmetry of corticomotor functional connectivity (Ackerley et al., 2016). Overall, in agreement with previous studies in healthy younger adults and individuals with clinical conditions, our findings suggest that alterations in cortical excitability in response to iTBS could be partially explained by changes in rsFC.
Baseline functional connectivity measures correlated with the enhancement in cortical excitability
Response heterogeneity has been reported in rTMS research (Bhandari et al., 2016). In this study, we examined the relationship between baseline rsFC and the subsequent iTBS aftereffects in older adults. We observed that baseline interhemispheric rsFC was associated with the responsiveness to iTBS in older adults. Specifically, stronger baseline interhemispheric rsFC between the sensorimotor cortex and premotor area was associated with reduced changes in cortical excitability after iTBS.
Previous fMRI studies have suggested that older adults tend to exhibit a greater spread of cortical activation during motor and cognitive processing compared with younger adults (Logan et al., 2002; Riecker et al., 2006), as well as a reduction of interhemispheric asymmetry in terms of activation (Cabeza, 2002). We speculate that stronger baseline interhemispheric rsFC between the sensorimotor cortex and premotor area during resting state might be associated with nonselective recruitment of brain activity in older adults. Different levels of interhemispheric communication during resting state might contribute to the increased variability of rsFC as well as response heterogeneity to iTBS in older adults.
These differences in baseline interhemispheric rsFC among older adults may have significant implications for the design of individualized TBS intervention. Older adults with stronger interhemispheric rsFC exhibit a narrower margin of improvement in rsFC after iTBS. This may be because the interhemispheric rsFC has been optimized as a compensatory mechanism in these older adults. Conversely, older adults with relatively low baseline interhemispheric rsFC have a greater margin for optimizing their functional brain patterns after iTBS.
In addition, the increased interhemispheric rsFC during resting state could be attributed to morphometric brain changes in older adults. Corpus callosum is the largest white matter bundle of commissural fibers between the hemispheres and likely plays a role in preserving functional asymmetry between homologous cortical regions (Bloom and Hynd, 2005; Roland et al., 2017; Rosen et al., 2013; Voineskos et al., 2010). The age-related differences in cortical activation and alteration in morphometric measurements were not reported in our study due to the limitation of lacking a younger group; there are reports in the literature that highlighted the possibility of contributing to more variability in interhemispheric connectivity patterns relative to younger adults.
Limitations and future directions
There were limitations that should be considered when interpreting the findings. One limitation of this study was the lack of a group of younger adults, as baseline rsFC in the younger group can provide more insight on age-related rTMS responsiveness. Second, we did not collect behavioral response with respect to motor task performance. Questions such as whether iTBS has an impact on motor accuracy or activity frequency and how the motor task performance is associated with rsFC should be addressed in future studies. Third, a sham-controlled design with a larger sample size in future studies would be warranted. Due to the relatively small sample size, the association between baseline interhemispheric rsFC and iTBS response should be interpreted with caution, and replication studies would be needed.
Fourth, participants were instructed to contract their right APB thumb muscle during the aIO curve measurements. This voluntary muscle activity in proximity to the application of iTBS over the primary motor cortex could modulate the aftereffects of iTBS. While the full mechanisms are not entirely elucidated, this is thought to be reflective of homeostatic metaplasticity as the voluntary contraction alters the synaptic activation history of the targeted cortical tissue, which in turn makes it more or less responsive to LTP/LTD-like responses (Goldsworthy et al., 2012, 2014, 2015; Huang et al., 2008). It is possible that metaplasticity is influencing our findings in ways that were not fully captured in our data set, and this is a limitation of the study.
For future suggestions, other individual and momentary features related to brain state and morphology as well as gene polymorphism have been shown to moderate the rTMS effects (Cheeran et al., 2008). Therefore, it will be essential to incorporate these potential moderating factors such as white matter integrity and genotype (e.g., brain-derived neurotrophic factor genotype) into future TMS studies. Given that baseline interhemispheric rsFC in the sensory motor and premotor regions is associated with iTBS-induced response, future studies should leverage baseline rsFC and develop personalized TMS protocols tailored for different individuals.
Conclusions
In summary, excitatory iTBS over the primary motor cortex can enhance cortical excitability and rsFC among sensory motor and premotor cortex in older adults. The responsiveness of iTBS is strongly correlated with changes in resting-state interhemispheric functional connectivity. Furthermore, older adults with higher baseline interhemispheric functional connectivity among the sensorimotor cortex and premotor regions exhibit reduced changes in cortical excitability after iTBS. Interhemispheric rsFC has great potential serving as a useful marker for future iTBS studies to account for iTBS responsiveness.
Supplementary Material
Acknowledgments
The authors express their gratitude to the research participants and to the reviewers for their insightful feedback.
Authors' Contributions
Y.L., K.L., M.H.S., S.C., and Y.-H.C. contributed to conceptualization; Y.L., K.L., M.H.S., C.U., S.C., and Y.-H.C. imparted methodology; M.H.S., K.L., and V.T.T. performed data collection; Y.L., K.L., M.H.S., and C.U. performed data analysis; Y.L., K.L., M.H.S., C.U., V.T.T., S.C., and Y.-H.C. contributed to writing, review, and editing; Y.-H.C. performed supervision and funding acquisition.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the National Institutes of Health R01 AG062543 (Principal Investigator [PI]: Y.-H.C.) and R21 AG077153 (PI: Y.-H.C.).
Supplementary Material
References
- Ackerley SJ, Byblow WD, Barber PA, et al. . 2016. Primed physical therapy enhances recovery of upper limb function in chronic stroke patients. Neurorehabil Neural Repair 304:339–348. [DOI] [PubMed] [Google Scholar]
- Barkhof F, Haller S, Rombouts SARB. 2014. Resting-state functional MR imaging: a new window to the brain. Radiology 2721:29–49. [DOI] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD. 2012. Evidence for motor cortex dedifferentiation in older adults. Neurobiol Aging 339:1890–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuzon G, Timour Q, Saoud M. 2017. Predictors of response to repetitive transcranial magnetic stimulation rTMS in the treatment of major depressive disorder. L'Encéphale 431:3–9. [DOI] [PubMed] [Google Scholar]
- Bhandari A, Radhu N, Farzan F, et al. . 2016. A meta-analysis of the effects of aging on motor cortex neurophysiology assessed by transcranial magnetic stimulation. Clin Neurophysiol 1278:2834–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom JS, Hynd GW. 2005. The role of the corpus callosum in interhemispheric transfer of information: excitation or inhibition? Neuropsychol Rev 152:59–71. [DOI] [PubMed] [Google Scholar]
- Cabeza R. 2002. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging 171:85–100. [DOI] [PubMed] [Google Scholar]
- Cárdenas-Morales L, Volz LJ, Michely J, et al. . 2014. Network connectivity and individual responses to brain stimulation in the human motor system. Cereb Cortex 247:1697–1707. [DOI] [PubMed] [Google Scholar]
- Cheeran B, Talelli P, Mori F, et al. . 2008. A common polymorphism in the brain-derived neurotrophic factor gene BDNF modulates human cortical plasticity and the response to rTMS. J Physiol 586:5717–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y hui, You H, Wang H, et al. . 2015. Effect of repetitive transcranial magnetic stimulation on fMRI resting-state connectivity in multiple system atrophy. Brain Connect 57:451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B, McGregor KM, Nocera JR, et al. . 2015. The relevance of aging-related changes in brain function to rehabilitation in aging-related disease. Front Hum Neurosci 9:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng ZD, Lisanby SH, Peterchev AV. 2013. Electric field depth-focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimulat 61:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denslow S, Lomarev M, George MS, et al. . 2005. Cortical and subcortical brain effects of transcranial magnetic stimulation (TMS)-induced movement: an interleaved TMS/functional magnetic resonance imaging study. Biol Psychiatry 57:752–760. [DOI] [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. 1997. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res 1142:329–338. [DOI] [PubMed] [Google Scholar]
- Dunlop K, Woodside B, Olmsted M, et al. . 2016. Reductions in cortico-striatal hyperconnectivity accompany successful treatment of obsessive-compulsive disorder with dorsomedial prefrontal rTMS. Neuropsychopharmacology 415:1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figee M, Luigjes J, Smolders R, et al. . 2013. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci 164:386–387. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Zackowski KM, Huey KA, et al. . 1993. Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J Physiol 460:549–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersner R, Kravetz E, Feil J, et al. . 2011. Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: differential outcomes in anesthetized and awake animals. J Neurosci 3120:7521–7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godde B, Berkefeld T, David-Jürgens M, et al. . 2002. Age-related changes in primary somatosensory cortex of rats: evidence for parallel degenerative and plastic-adaptive processes. Neurosci Biobehav Rev 267:743–752. [DOI] [PubMed] [Google Scholar]
- Goh JOS. 2011. Functional dedifferentiation and altered connectivity in older adults: neural accounts of cognitive aging. Aging Dis 21:30–48. [PMC free article] [PubMed] [Google Scholar]
- Goldsworthy MR, Müller-Dahlhaus F, Ridding MC, et al. . 2014. Inter-subject variability of LTD-like plasticity in human motor cortex: a matter of preceding motor activation. Brain Stimulat 7:864–870. [DOI] [PubMed] [Google Scholar]
- Goldsworthy MR, Müller-Dahlhaus F, Ridding MC, et al. . 2015. Resistant against de-depression: LTD-like plasticity in the human motor cortex induced by spaced cTBS. Cereb Cortex 25:1724–1734. [DOI] [PubMed] [Google Scholar]
- Goldsworthy MR, Pitcher JB, Ridding MC. 2012. The application of spaced theta burst protocols induces long-lasting neuroplastic changes in the human motor cortex. Eur J Neurosci 35:125–134. [DOI] [PubMed] [Google Scholar]
- Guerra A, López-Alonso V, Cheeran B, et al. . 2020. Variability in non-invasive brain stimulation studies: reasons and results. Neurosci Lett 719:133330. [DOI] [PubMed] [Google Scholar]
- Hamada M, Murase N, Hasan A, et al. . 2013. The role of interneuron networks in driving human motor cortical plasticity. Cereb Cortex 237:1593–1605. [DOI] [PubMed] [Google Scholar]
- Hinder MR, Goss EL, Fujiyama H, et al. . 2014. Inter- and intra-individual variability following intermittent theta burst stimulation: implications for rehabilitation and recovery. Brain Stimul Basic Transl Clin Res Neuromodulation 73:365–371. [DOI] [PubMed] [Google Scholar]
- Hoogendam JM, Ramakers GMJ, Lazzaro VD. 2010. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul Basic Transl Clin Res Neuromodulation 32:95–118. [DOI] [PubMed] [Google Scholar]
- Hordacre B, Goldsworthy MR, Vallence AM, et al. . 2017. Variability in neural excitability and plasticity induction in the human cortex: a brain stimulation study. Brain Stimul Basic Transl Clin Res Neuromodulation 103:588–595. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, et al. . 2005. Theta burst stimulation of the human motor cortex. Neuron 452:201–206. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Edwards MJ, et al. . 2008. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex 18:563–570. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, et al. . 2012. FSL. Neuroimage 622:782–790. [DOI] [PubMed] [Google Scholar]
- Jockwitz C, Caspers S. 2021. Resting-state networks in the course of aging—differential insights from studies across the lifespan vs. amongst the old. Pflüg Arch Eur J Physiol 4735:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Bungert A, Bowtell R, et al. . 2020. Modulating brain networks with transcranial magnetic stimulation over the primary motor cortex: a concurrent TMS/fMRI study. Front Hum Neurosci 14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenberg LAC, Schulte E, Disselhorst-Klug C, et al. . 2007. Myoelectric manifestations of fatigue at low contraction levels in subjects with and without chronic pain. J Electromyogr Kinesiol 17:264–274. [DOI] [PubMed] [Google Scholar]
- Langan J, Peltier SJ, Bo J, et al. . 2010. Functional implications of age differences in motor system connectivity. Front Syst Neurosci 4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Park E, Lee A, et al. . 2018. Modulating brain connectivity by simultaneous dual-mode stimulation over bilateral primary motor cortices in subacute stroke patients. Neural Plast 2018:e1458061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, et al. . 2002. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron 335:827–840. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Gottlob LR, Allen PA. 1999. Adult age differences in visual search accuracy: attentional guidance and target detectability. Psychol Aging 144:683–694. [DOI] [PubMed] [Google Scholar]
- McGregor KM, Zlatar Z, Kleim E, et al. . 2011. Physical activity and neural correlates of aging: a combined TMS/fMRI study. Behav Brain Res 2221:158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min YS, Park JW, Jin SU, et al. . 2016. Neuromodulatory effects of offline low-frequency repetitive transcranial magnetic stimulation of the motor cortex: a functional magnetic resonance imaging study. Sci Rep 6:36058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkova L, Peter J, Abdulkadir A, et al. . 2019. Determinants of inter-individual variability in corticomotor excitability induced by paired associative stimulation. Front Neurosci 13:841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisa M, Pohmann R, Ewald L, et al. . 2009. New coil positioning method for interleaved transcranial magnetic stimulation TMS/functional MRI fMRI and its validation in a motor cortex study. J Magn Reson Imaging 291:189–197. [DOI] [PubMed] [Google Scholar]
- Naito E, Roland PE, Ehrsson HH. 2002. I feel my hand moving: a new role of the primary motor cortex in somatic perception of limb movement. Neuron 36:979–988. [DOI] [PubMed] [Google Scholar]
- Nettekoven C, Volz LJ, Leimbach M, et al. . 2015. Inter-individual variability in cortical excitability and motor network connectivity following multiple blocks of rTMS. Neuroimage 118:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinemann A, Lehner C, Conrad B, et al. . 2001. Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neurosci Lett 3131:33–36. [DOI] [PubMed] [Google Scholar]
- Pell GS, Roth Y, Zangen A. 2011. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: influence of timing and geometrical parameters and underlying mechanisms. Prog Neurobiol 931:59–98. [DOI] [PubMed] [Google Scholar]
- Riecker A, Gröschel K, Ackermann H, et al. . 2006. Functional significance of age-related differences in motor activation patterns. Neuroimage 323:1345–1354. [DOI] [PubMed] [Google Scholar]
- Roland JL, Snyder AZ, Hacker CD, et al. . 2017. On the role of the corpus callosum in interhemispheric functional connectivity in humans. Proc Natl Acad Sci U S A 11450:13278–13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland PE, O'Sullivan B, Kawashima R. 1998. Shape and roughness activate different somatosensory areas in the human brain. Proc Natl Acad Sci U S A 95:3295–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen A, Stephens J, Glover G, et al. . 2013. P 198. Relationship between functional connectivity and interhemispheric inhibition in older adults. Clin Neurophysiol 12410:e158–e159. [Google Scholar]
- Rossini PM, Burke D, Chen R, et al. . 2015. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 1266:1071–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Llonch R, Bartrés-Faz D, Junqué C. 2015. Reorganization of brain networks in aging: a review of functional connectivity studies. Front Psychol 6:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Dunlop K, Kennedy SH, et al. . 2014. Resting-state cortico-thalamic-striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacology 392:488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Huang P, Li Y, et al. . 2019. Age-related changes in the plasticity of neural networks assessed by transcranial magnetic stimulation with electromyography: a systematic review and meta-analysis. Front Cell Neurosci 13:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecchio F, Zappasodi F, Pasqualetti P, et al. . 2008. Age dependence of primary motor cortex plasticity induced by paired associative stimulation. Clin Neurophysiol 1193:675–682. [DOI] [PubMed] [Google Scholar]
- Tik M, Hoffmann A, Sladky R, et al. . 2017. Towards understanding rTMS mechanism of action: stimulation of the DLPFC causes network-specific increase in functional connectivity. Neuroimage 162:289–296. [DOI] [PubMed] [Google Scholar]
- Todd G, Kimber TE, Ridding MC, et al. . 2010. Reduced motor cortex plasticity following inhibitory rTMS in older adults. Clin Neurophysiol 1213:441–447. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. . 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 151:273–289. [DOI] [PubMed] [Google Scholar]
- Ueno S, Tashiro T, Harada K. 1988. Localized stimulation of neural tissues in the brain by means of a paired configuration of time-varying magnetic fields. J Appl Phys 6410:5862–5864. [Google Scholar]
- Voineskos AN, Farzan F, Barr MS, et al. . 2010. The role of the corpus callosum in transcranial magnetic stimulation induced interhemispheric signal propagation. Biol Psychiatry 689:825–831. [DOI] [PubMed] [Google Scholar]
- Volz LJ, Benali A, Mix A, et al. . 2013. Dose-dependence of changes in cortical protein expression induced with repeated transcranial magnetic theta-burst stimulation in the rat. Brain Stimul Basic Transl Clin Res Neuromodulation 64:598–606. [DOI] [PubMed] [Google Scholar]
- Volz LJ, Hamada M, Rothwell JC, et al. . 2015. What makes the muscle twitch: motor system connectivity and TMS-induced activity. Cereb Cortex 259:2346–2353. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Hanajima R, Shirota Y, et al. . 2013. Bidirectional effects on interhemispheric resting-state functional connectivity induced by excitatory and inhibitory repetitive transcranial magnetic stimulation. Hum Brain Mapp 355:1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Besser L, Dodge HH, et al. . 2018. Version 3 of the Alzheimer Disease Centers' Neuropsychological Test Battery in the Uniform Data Set UDS. Alzheimer Dis Assoc Disord 321:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. 2012. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 23:125–141. [DOI] [PubMed] [Google Scholar]
- Wischnewski M, Schutter DJLG. 2015. Efficacy and time course of theta burst stimulation in healthy humans. Brain Stimulat 84:685–692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.