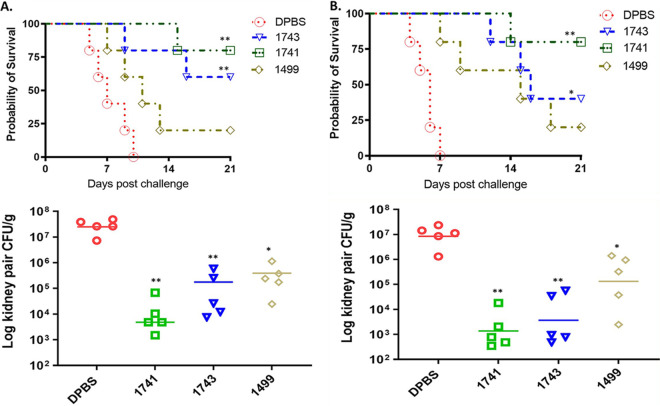
FIG 1.
Passive transfer of human IVIG samples from healthy donors can provide protection against disseminated C. auris and C. albicans infections in mice. Based on the endpoint titers of specific IgGs, we first investigated the preventative efficacy of three IVIG lots in protection against C. auris disseminated infections in an A/J mouse model (A) and C. albicans disseminated infections in an immunosuppressed C57BL/6 mouse model (200-mg/kg dose of cyclophosphamide intraperitoneally [i.p.] given weekly) (B). We performed i.p. injections of each selected IVIG lot into mice 4 h before lethal intravenous challenge with C. auris (AR-CDC0386, a human clinical isolate from South American clade IV) (2 × 108 CFU) or C. albicans (SC5314) (1 × 106 CFU) a reference strain, which is a clinical isolate. IVIG batch 1741, containing the highest IgG titers against all five Candida cell surface epitopes, provided the best protection (60 to 80% survival), with significantly prolonged survival and greatly reduced CFU against both C. auris and C. albicans (P < 0.01). Data are means and SD (n = 5). A log rank (Mantel-Cox) test and a two-tailed t test were used to identify significant differences (**, P < 0.01; *, P < 0.05). For Candida invasive infection, A/J mice (female, 5 to 7 weeks of age) or C57BL/6 mice (female, 5 to 7 weeks of age) were treated with DPBS or each IVIG sample 4 h before lethal challenge with each Candida isolate. The mice were monitored for survival up to 21 days postchallenge, and CFU were evaluated in the kidney either when mice succumbed to the disease or at termination on day 21 postinfection (p.i.).