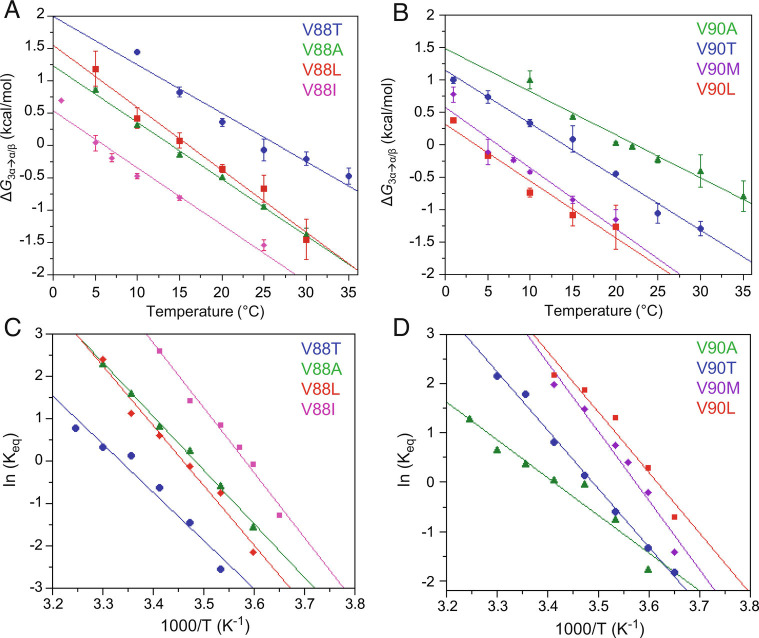
Fig. 6.
Energetics of fold interconversion between 3α and α/β-plait structures. (A) ΔG3α→α/β versus temperature for the Sa1 mutants V88T (blue), V88A (green), V88L (red), and V88I (magenta). (B) ΔG3α→α/β versus temperature for Sa1 mutants V90A (green), V90T (blue), V90L (red), and V90M (purple). ΔG3α→α/β is calculated for V88 and V90 mutants from the equilibrium constant for exchange between 3α and α/β-plait conformations, K3α→α/β, at temperatures of 1 to 35 °C using the equation ΔG3α→α/β = −RTln K3α→α/β. The equilibrium constant at each temperature is an average value determined from peak volumes for main chain amide signals of 4 to 6 residues that are in the folded regions of both 3α and α/β-plait forms and have similar relaxation properties in both states. (C) Van’t Hoff plots of logKeq versus 1/T for the Sa1 mutants V88T (blue), V88A (green), V88L (red), and V88I (magenta). (D) Van’t Hoff plots for the Sa1 mutants V90A (green), V90T (blue), V90L (red), and V90M (purple). The plots in (C and D) are fitted to the linear form of the Van’t Hoff equation (Eq. 1), which is used to estimate the enthalpy and entropy of the fold conversion from the slope and intercept, respectively.