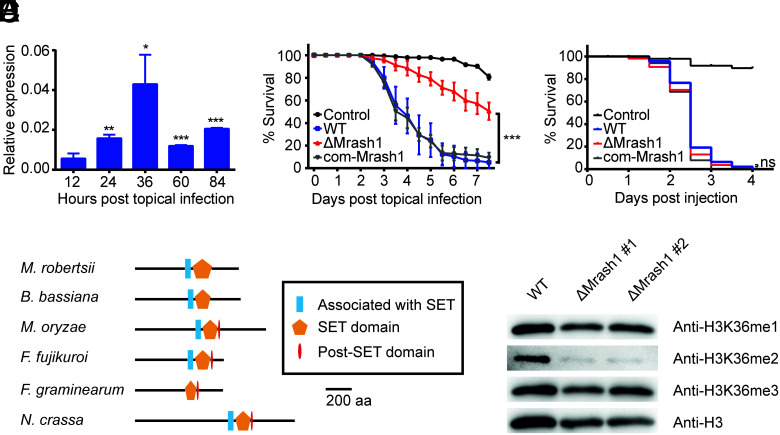
Fig. 1.
MrASH1, an H3K36me2 methyltransferase in M. robertsii, affects fungal pathogenicity. (A) qPCR analysis of Mrash1 transcription during topical infection of A. stephensi by the WT strain. Data are shown as the mean ± SD of three technical replicates. Significant differences compared with that at 12 h after topical infection were determined by Student's t test. *P < 0.05, **P < 0.01, and ***P < 0.001. gpd was used as a reference gene. The experiments were repeated twice with similar results. (B) Survival of female adult A. stephensi mosquitoes following topical application of conidial suspension (6 × 106 conidia/ml) of the WT, ΔMrash1, and com-Mrash1 strains. The control mosquitoes were treated with 0.01% Triton X-100. Each treatment was replicated three times, with 50 mosquitoes per replicate. Significant differences compared with those in WT were determined by the log-rank (Mantel–Cox) test. ***P < 0.001. The experiments were repeated twice with similar results. (C) Survival of female adult A. stephensi mosquitoes after injection of conidial suspension (138 nL of 1 × 106 conidia/mL) of the WT, ΔMrash1, and com-Mrash1 strains. The control mosquitoes were injected with 0.01% Triton X-100 in PBS. Fifty mosquitoes were used in each treatment. Significant differences compared with those in WT were determined by the log-rank (Mantel–Cox) test. ns, not significant. (D) Protein domain structures of MrASH1 and its homologues in other fungal species. aa, amino acid. (E) Western blotting analysis of histone modifications in the WT and two ΔMrash1 mutants. Histones extracted from M. robertsii mycelia grown in MM2 medium were resolved by 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and probed with specific antibodies against H3K36me1, H3K36me2, H3K36me3, and a C-terminal peptide of histone H3.