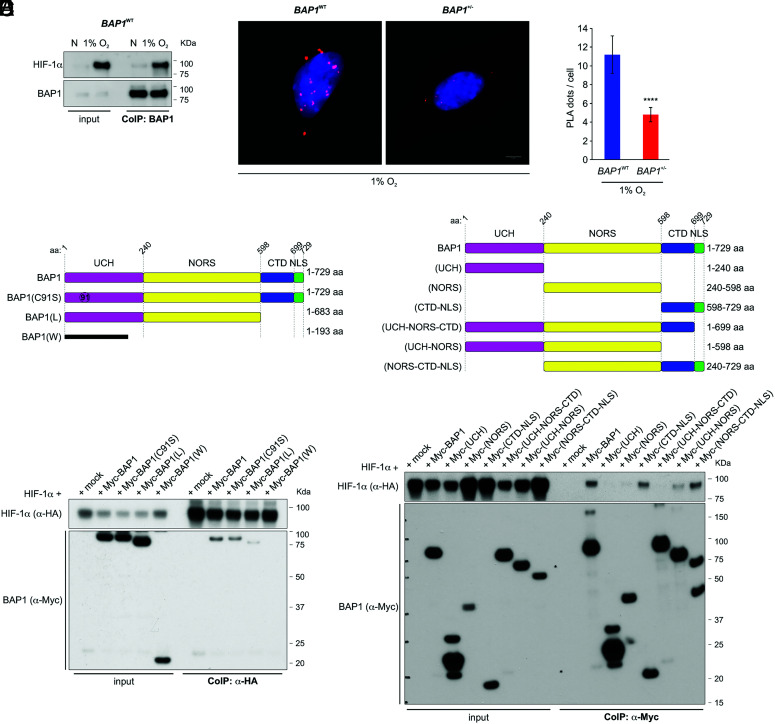
Fig. 2.
BAP1 binds HIF-1α. (A) HIF-1α and BAP1 co-precipitate. CoIP of endogenous HIF-1α and BAP1, in BAP1WT fibroblasts grown in normoxia (N) or hypoxia (1% O2) for 4 h, using BAP1 as a bait. (B and C) PLA: red dots demonstrate the BAP1–HIF-1α interaction in the nuclei of BAP1WT and BAP1+/− fibroblasts incubated in 1% O2 for 6 h. Nuclei stained blue with DAPI (B); (Scale bar: 5 μm.) Bar graph: quantification of PLA red dots per cell showing reduced BAP1–HIF-1α interaction in BAP1+/− fibroblasts. Data shown as mean ± SD (n = 20 cells per condition) (C). (D and E) Mapping of the BAP1–HIF-1α interaction. The deletion of the CTD-NLS BAP1 domain –as observed in individuals of the W and L families, greatly reduces the interaction with HIF-1α. (D) CoIP of HIF-1α and BAP1 in homogenates from HEK-293 co-transfected with HA-tagged HIF-1α and Myc-tagged [displayed on top (4)], BAP1, catalytic inactive (C91S), L family truncated mutant, W family truncated mutant, using anti-HA resin (E) The CTD-NLS domain of BAP1 is the major contributor to the interaction with HIF-1α, while the fragment consisting of the UCH together with the NORS domains binds to a minor extent. CoIP of HIF-1α and BAP1 in homogenates from HEK-293 co-transfected with HA-tagged HIF-1α and Myc-tagged BAP1, and the Myc-tagged BAP1 fragments displayed on top (4) using anti-Myc resin.