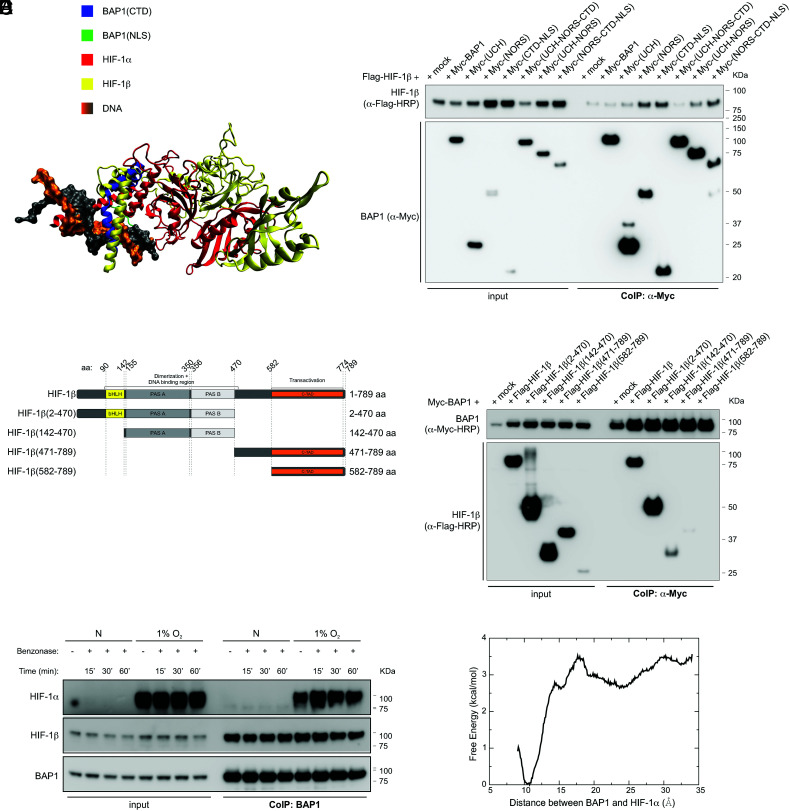
Fig. 4.
BAP1 binding to HIF-1α and HIF-1β does not require DNA. (A) Shared binding region among BAP1, HIF-1α, and HIF-1β. The CTD of BAP1 is colored in blue, the NLS domain of BAP1 is colored in green, HIF-1α is colored in red, DNA is colored in orange and grey, and HIF-1β (colored in yellow) is docked onto the binding complex of BAP1 and HIF-1α by utilizing the crystal structure of HIF-1α–HIF-1β (PDB ID: 4zpr) (49). Missing residues of the crystal structure are added by the SWISS-MODEL server (50). (B) HIF-1β interacts with the NORS and CTD-NLS domain of BAP1. CoIP of HIF-1β and BAP1 in homogenates from HEK-293 co-transfected with Flag-tagged HIF-1β and Myc-tagged BAP1 and the Myc-tagged BAP1 fragments displayed in Fig. 2E (4), using anti-Myc resin. (C) Flag-tagged HIF-1β fragments and HIF-1β domains: basic-helix-loop-helix motif (bHLH) protein, two Per and Sim (PAS) domain (A and B), and COOH-terminal transactivation domain (C-TAD). (D) CoIP of BAP1 and HIF-1β in homogenates from HEK-293 co-transfected with Myc-BAP1 and Flag-HIF-1β or the Flag-HIF-1β fragments displayed in (C), or the empty vector (mock); anti-Myc resin was used as bait. (E) HEK-293 cells were grown in normoxia (N) or hypoxia (1% O2) for 4 h. Cell homogenates were collected, treated with benzonase for 15, 30 or 60 min (SI Appendix, Fig. S3), and then used to co-immunoprecipitate endogenous HIF-1α and HIF-1β using BAP1 as bait. (F) Computational binding free energy profile between BAP1 and HIF-1α in the absence of DNA; the result indicates that the binding complex formed by BAP1 and HIF-1α can still hold when DNA is absent (the binding free energy ~ 3 kcal/mol).