TDP-43 is a DNA/RNA-binding protein involved in numerous neurodegenerative diseases, including Alzheimer’s Disease (AD), and physiological aging [3]. TDP-43 pathology affects memory and cognition [4]. Between 1999 and 2021, Mayo Clinic has amassed a database with 1072 participants with known TDP-43 pathology status and AD neuropathological changes, who were prospectively recruited and followed in the NIH-funded Mayo Clinic Alzheimer’s Disease Research Center, Mayo Clinic Study of Aging, and Neurodegenerative Research Group, and underwent neurologic, genetic, and neuropathologic evaluations. Participants diagnosed with frontotemporal lobar degeneration with TDP-43 (FTLD-TDP)[2] postmortem were excluded. Amygdala sections were screened to determine TDP-43 status, as previously described [3]. Cases showing TDP-43-immunoreactive inclusions typically seen in FTLD-TDP (type-α) and/or colocalizing with neurofibrillary tangles (type-β)[2] were designated TDP-43 positive(+) and subsequently staged using the Josephs 6-stage TDP-43 distribution scheme in AD [3]. AD neuropathological assessment was based on the NIA-Reagan Institute or NIA-Alzheimer’s Association criteria. Hippocampal sclerosis, Lewy body disease, amyloid angiopathy, arteriolosclerosis, large and microinfarcts were assessed following established guidelines.
Our cohort was White (99%) with a median age at death of 87 years (IQR:80–92) and even sex distribution (52% female). Ninety-eight percent (1052/1072) had an antemortem, consensus-based cognitive state diagnosis determined through standardized neuropsychological and clinical assessments, 58% of whom had dementia of broad type, 15% had mild impairment, and 27% were unimpaired. Characteristics of the cohort and the subsets with dementia (n=611) and without dementia (n=441) are shown in Table 1, online resource. Fifty-two percent of the cohort had intermediate-high ADNC. TDP-43-immunoreactivity was detected in 44% (470/1072) of the cohort, 53% of those with dementia and 32% of those without. Only 4/326 (1%) TDP-43(+) participants with dementia had pure-TDP-43 pathology (Table 2, online resource). Ongoing staging was hitherto completed for 361/470 [3]. The median TDP-43 stage was 3 in both cohort (IQR:1–5) and dementia subgroup (IQR:2–5), but was lower (median=2, IQR:1–5) in the no-dementia subgroup.
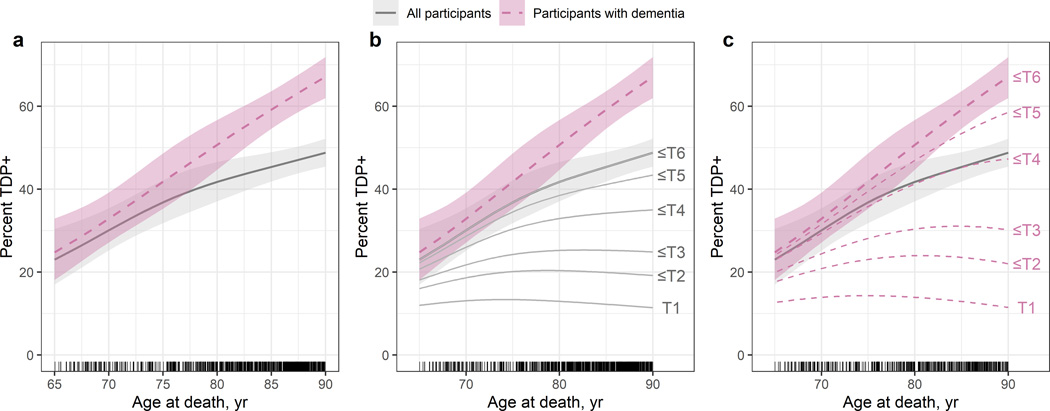
TDP-43 pathology frequency by age at death was estimated using logistic regression with age modelled using a restricted cubic spine to allow for a flexible functional form. Models were fit for all cases and for the dementia subgroup. TDP-43(+) frequency increased approximately linearly with age at death in both cohort and dementia subgroup. However, estimated frequencies in the dementia subgroup were higher at all ages compared to the cohort and the increase in frequency over time occurred at a faster rate (Fig.1a). At 70 years at death, an estimated 30%(95%CI:25%–35%) of the cohort was TDP-43(+) versus 33%(CI:27%–39%) of those with dementia. At 80 years, 42%(CI:37%–47%) of the cohort was TDP-43(+) versus 51%(CI:45%–57%) of dementia subgroup. By 90 years, 49%(CI:45%–52%) of the cohort and 67%(CI:62%–72%) of those with dementia were TDP-43(+).
Fig.1:
The estimated frequencies of TDP-43(+) by age at death across all subjects and the subset with dementia are plotted with 95% confidence intervals (A). The cumulative frequencies of the 6 TDP-43 stages in the cohort (B) and the dementia subgroup (C) are shown. Tick marks above the x-axis reflect individual patient points.
To estimate the frequency of each stage among the TDP-43(+) as a function of age, we fit an ordinal logistic regression model using the 361 total participants with TDP-43 stage and the 271 participants with TDP-43 stage and dementia. In both the cohort (Fig.1b) and dementia subgroup (Fig.1c), the frequencies of TDP-43 stages 1–3 were relatively constant beginning age 75. Conversely, the frequencies of TDP-43 stages extending beyond the hippocampus (stages 4–6) were higher and increased more rapidly in the dementia subgroup compared to the cohort particularly beginning age 80, where notable differences started to appear and continued to increase through age 90.
The results from our large cohort confirm that TDP-43 pathology frequency increases in elderly White participants with age of death, independent of cognitive status [4]. We further demonstrate that TDP-43 pathology frequency increases to a greater degree and at a faster rate with age in those with dementia. Given the clear links between TDP-43 and impaired cognition, the contribution of TDP-43 pathology to an individual’s cognitive impairment is likely to increase with age. This dynamic will have significant public health consequences given our aging population. However, factors other than TDP-43-immunoreactivity are also likely to contribute. Indeed, 30% of our TDP-43(+) participants were either cognitively normal or mildly impaired. One factor could be the TDP-43 stage since the frequency of TDP-43 pathology extending beyond the hippocampus (stage4) was higher and increased at a greater rate in those with dementia, suggesting that stage is playing a key role in severe cognitive impairment. This coincidentally highlights the importance of the 6-stage scheme. Another factor could be TDP-43 type given that resilience occurs with TDP-43 type-β, but not type-α [1]. Additionally, 1% of our demented TDP-43(+) participants had pure-TDP-43, implying that co-pathologies are pivotal. While the exact relationships between TDP-43 pathology and co-pathologies remain indistinct, any tangible “TDP-43 effect” can be expected to escalate with increasing age, reflecting its increasing frequency. Our findings suggest that TDP-43 pathology is common in dementia, and its complex relationship with cognition and age warrants further evaluation.
Supplementary Material
References:
- 1.Buciuc M, Whitwell JL, Tosakulwong N, Weigand SD, Murray ME, Boeve BF, Knopman DS, Parisi JE, Petersen RC, Dickson DW et al. (2020) Association between transactive response DNA-binding protein of 43 kDa type and cognitive resilience to Alzheimer’s disease: a case-control study. Neurobiol Aging 92: 92–97 Doi 10.1016/j.neurobiolaging.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Josephs KA, Murray ME, Tosakulwong N, Weigand SD, Serie AM, Perkerson RB, Matchett BJ, Jack CR Jr., Knopman DS, Petersen RC et al. (2019) Pathological, imaging and genetic characteristics support the existence of distinct TDP-43 types in non-FTLD brains. Acta Neuropathol 137: 227–238 Doi 10.1007/s00401-018-1951-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Josephs KA, Murray ME, Whitwell JL, Tosakulwong N, Weigand SD, Petrucelli L, Liesinger AM, Petersen RC, Parisi JE, Dickson DW (2016) Updated TDP-43 in Alzheimer’s disease staging scheme. Acta Neuropathol 131:571–585 Doi 10.1007/s00401-016-1537-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson RS, Yu L, Trojanowski JQ, Chen EY, Boyle PA, Bennett DA, Schneider JA (2013) TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol 70: 1418–1424 Doi 10.1001/jamaneurol.2013.3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.