Abstract
The objective was to evaluate efficacy of lymphosonography to identify sentinel lymph nodes (SLNs) in breast cancer patients undergoing surgical excision. 86 subjects were enrolled and 79 completed this IRB-approved study. Subjects received subcutaneous 1.0mL injections of ultrasound contrast agent (UCA) around the tumor. Ultrasound scanner with contrast-enhanced ultrasound (CEUS) capabilities was used to identify SLNs. Subjects received blue dye and radioactive tracer for guiding SLN excision as standard-of-care. Excised SLNs were classified as positive or negative for presence of blue dye, radioactive tracer and UCA, and sent for pathology. 252 SLNs were excised, 158 positive for blue dye, 222 positive for radioactive tracer and 223 positive for UCA. Comparison to blue dye showed accuracy of 96.2% for radioactive tracer and 99.4% for lymphosonography (p>0.15). Relative to radioactive tracer, blue dye showed accuracy of 68.5%, while lymphosonography achieved 86.5% (p<0.0001). Of 252 SLNs excised, 34 were determined malignant by pathology; 18 positive for blue dye (detection rate 53%), 23 positive for radioactive tracer (detection rate 68%) and 34 positive for UCA (detection rate 100%; p<0.0001). Lymphosonography achieved similar accuracy as radioactive tracer and higher accuracy than blue dye for identifying SLNs. All 34 malignant SLNs were identified by lymphosonography.
Keywords: Contrast-enhanced ultrasound, sentinel lymph node, breast cancer, lymphatic tracer, ultrasound contrast agent, lymphosonography, lymphatic mapping, ultrasound
Introduction
The sentinel lymph node (SLN) concept theorizes that metastatic cells spread through the lymphatic system with the SLN being the first one in the lymphatic chain (Boughey et al. 2015; Emerson et al. 2012; Tokin et al. 2012; Tan et al. 2011). Therefore, if the SLN is free of cancer cells the rest of the lymphatic chain is also without metastatic disease (Emerson et al. 2012; Tokin et al. 2012). The map of the lymphatic chain is clinically important for predicting long-term outcomes for patients with breast cancer and currently is obtained after the injection of blue dye and/or a radioactive tracer followed by surgical excision (Emerson et al. 2012; Hill et al. 1999; Eshima et al. 2000; Veronesi et al. 2003).
These approaches, however, have wide variability in the accuracy for detection of SLNs ranging from 76% to 97% (Veronesi et al. 2003; Camp et al. 2004; Cabioğlu et al. 2018; Jung et al. 2019; Kinoshita et al. 2006; Kang et al. 2004). There are limitations and potential adverse experiences (AEs) for the techniques, such as an unnecessary extensive surgical dissection if the tracers drain into secondary and/or tertiary lymph nodes (LN); hence, the injection of blue dye and/or radioactive tracer needs to be done shortly before the surgical procedure to try to limit the extension of the tracer through the lymphatic system (Goldberg et al. 2005; Goldberg et al. 2011; Goldfarb et al. 1998; Hill et al. 1999). Blue dye can cause anaphylactic reactions having an overall incidence of allergic reactions of 1.8% with severe reactions in 0.2% and skin staining that can last up to one year after the injection (Wohrl et al. 2004; King et al. 2004; Govaert, Oostenbroek, and Plaisier 2005). The radioactive tracers use radiation, even if it is low dose it nonetheless involves exposure not only for the patient but also for the surgical team (Hill et al. 1999; Goldfarb et al. 1998; Goldberg et al. 2005; Goldberg et al. 2011).
Diagnostic ultrasound imaging (US) has been used to evaluate LNs for benign as well as metastatic disease and also as a valuable method to guide LN biopsies (Evans and Boyd 2007; Cho et al. 2009; Voit et al. 2009). Conventional US modes such as grayscale, color Doppler and power Doppler (combined or individually) are part of clinical patient care to determine the level of suspicious of a LN for malignancy (Evans and Boyd 2007; Cho et al. 2009; Voit et al. 2009; Romeo et al. 2021). However, US could not be used for lymphatic mapping, since mapping requires administration of a tracer. This paradigm changed when reports on the use of contrast-enhanced US (CEUS) to detect LCs and LNs after subdermal injections of microbubble-based US contrast agents (UCAs) in animal models and clinical trials (termed “lymphosonography”) were produced (Goldberg et al. 2011; Goldberg et al. 2005; Liu et al. 2014; Mattrey et al. 2002; Omoto et al. 2009; Sever et al. 2011; Sever, Mills, Jones, et al. 2012). Our group used a Sinclair swine model with naturally occurring melanomas for pre-clinical trials (Goldberg et al. 2004; Goldberg et al. 2005; Goldberg et al. 2011), which showed a detection rate of 82% for lymphosonography and 63% for lymphoscintigraphy (p<0.0001) (Goldberg et al. 2011). The translation to humans started with the development of clinical trials focused on patients with breast cancer where the UCA was injected around the areola using lymphosonography to follow the LCs to the axilla until the corresponding SLNs; results from these studies are promising albeit varied with detection rates from 70% to 100%, in concordance with the standard of care tracers currently in use (Omoto et al. 2009; Sever et al. 2011; Sever, Mills, Jones, et al. 2012; Sever, Mills, Weeks, et al. 2012; Cox et al. 2018; Cox et al. 2016; Cox et al. 2013). Moreover, our group studied the safety of lymphosonography in healthy volunteers and showed that the only AEs observed were minor, local and non-significant confined to the injection site and surrounding area and these AEs completely resolved without any intervention (Machado et al. 2018).
The next step for our group was the translation to a clinical trial where the technique would be evaluated in patients with breast cancer. Hence, the objective of this study was to evaluate the efficacy of CEUS lymphosonography in the identification of SLNs in patients with breast cancer undergoing surgical excision following the injection of blue dye and radioactive tracer as part of their standard of care using pathology results for malignancy as a reference standard.
To accomplish the above objective, our group developed a study protocol using subcutaneous UCA injections of Sonazoid around the tumor area in order to identify the precise lymphatic drainage region connected with the tumor. Sonazoid is a very stable as well as reticuloendothelial system specific UCA, which ensures that the microbubbles are eliminated by the macrophages and therefore becomes an intracellular UCA for a period of time [around 24h; (Goldberg et al. 2005; Machado et al. 2018)]. The SLN enhancement will last just as long.
Material and Methods
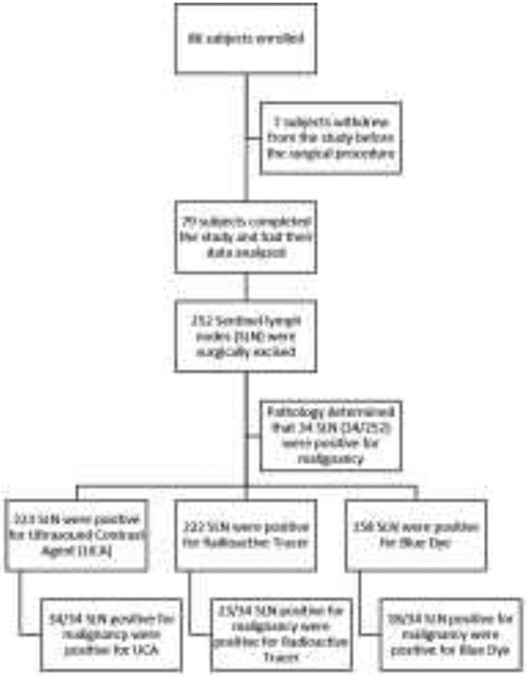
Eighty-six (86) women scheduled for breast cancer surgery with SLNs excision were enrolled and 79 completed the study between March, 2017 and January, 2022. The subjects went through a full demographic profile, known drug allergies or intolerances, and a review of theirs medical/surgical history. The inclusion criteria were female subjects diagnosed with breast cancer and scheduled for a surgical excision with SLN evaluation. Exclusion criteria were female subjects that were pregnant or breastfeeding or allergic to Sonazoid or with prior breast reduction surgery or subjects that had a prior axillary surgical procedure ipsilateral to the current tumor. Figure 1 shows a diagram of subject enrollment and procedures.
Figure 1:
Enrollment diagram
All enrolled subjects provided written informed consent. The study was approved by the University’s Institutional Review Board as well as the United States Food and Drug administration (IND no. 124,465), and was compliant with the Health Insurance Portability and Accountability Act. The full protocol and statistical analysis plan (including the power analysis) are available at https://clinicaltrials.gov/ct2/show/NCT02652923 (trial registration number: NCT02652923).
UCAs are gas filled microbubbles measuring 1-10 μm (similar to the red blood cells), where the gas is encapsulated with a protein, lipid or polymeric shell to increase stability (Lyshchik 2019). UCAs not only enhance the backscattered signals, but at higher acoustic pressures they also act as nonlinear oscillators producing significant energy components in the received echo signals. These nonlinear bubble echoes can be preferentially displayed in contrast-specific imaging modes such as harmonic imaging (Lyshchik 2019; Goldberg, Liu, and Forsberg 1994; Forsberg et al. 1998; Forsberg, Shi, and Goldberg 2000).
The subjects had the US examination done on the day of surgery prior to the radioactive tracer injection, the wire localization (when applicable) and of the surgery itself. The US examination consisted of B-mode of the tumor to determine location and size. Afterwards the UCA Sonazoid (GE Healthcare, Oslo, Norway), a reticuloendothelial specific agent (Goldberg et al. 2005; Goldberg et al. 2011), was subcutaneously injected around the tumor area at the 12,−3,−6,-and 9 o’clock positions (0.25 mL per position for a total of 1 mL). The injection sites were massaged for 5 minutes by experienced physicians (JBL, LN or PM) to accelerate the uptake of Sonazoid into the lymphatic system. CEUS was then performed (i.e., lymphosonography) to identify the number, location and course of the lymphatic channels (LCs) and SLNs using Cadence Pulse Sequencing (CPS; Siemens Healthineers, Mountain View, CA, USA) on an S3000 HELX scanner (Siemens Healthineers) with a high frequency, broad bandwidth (4 – 9 MHz) linear array probe (9L4). The mechanical index (MI) used to acquire the CEUS images was 0.12 to limit bubble destruction. Imaging parameters e.g., focal zone, scanning depth and time-gain compensation (TGC) were adjusted on an individual basis to optimize visualization of the target region (SLN or LC). No compounding or other image processing techniques were applied. Sagittal and transverse still images and digital clips were acquired during the US examination. The safety and tolerability of Sonazoid was closely monitored and any AEs were noted.
After the US examination, the subjects underwent standard of care prior to surgery: injection of radioactive tracer around the areola and when applicable (i.e., for lumpectomy) stereotactic wire localization of the tumor prior to the surgical procedure.
At the time of the surgical procedure when the subjects were already in the operating room (OR), they received injection of blue dye around the areola immediately prior to the beginning of surgery. After surgical excision of SLNs guided by both the blue dye and the radioactive tracer (standard of care), ex vivo SLN specimens were scanned using color Doppler imaging (CDI) to verify the uptake of the UCA (Sonazoid), since the higher acoustic energy transmitted in CDI mode causes the UCA microbubbles to burst, which can be seen as random color bursts or acoustic emissions (Forsberg et al. 2002).
All the excised ex vivo SLN specimens were classified as positive or negative for the presence of blue dye, radioactive tracer and UCA, while still in the OR by both the surgical and research teams. Finally, the ex vivo SLN specimens were sent to pathology to determine presence or absence of metastatic involvement.
Therefore, the study used a dual imaging confirmation method to determine that the same SLNs were seen before and during surgery. During the CEUS examination all SLNs had their location and measurements noted. The subject’s skin was not marked, because we did not want to bias the breast surgeons. In the operating room, the breast surgeons performed the standard of care SLN surgical excision. Once the SLN was removed from the surgical field we would perform an US examination pf the specimen using color Doppler to determine the UCA uptake by bursting the microbubbles. All excised SLNs had their locations, measurements, presence of UCA, presence of blue dye and presence of radioactive tracer noted. This information was then compared with the CEUS data, while the subject was still in the operation room to determine that they were the same SLNs. If there were any discrepancies they were noted and communicated to the breast surgeon, who decided if there were any CEUS identified SLNs not excised whether they would look for additional nodes based on their clinical judgement.
For data analysis the total number of SLNs excised were compared to the number of SLNs identified using lymphosonography, and the number of excised SLNs that were positive for blue dye, radioactive tracer and UCA. Results were compared between techniques and with the pathology findings using McNemar’s test. All tests were performed using Prism 9.3.1 (GraphPad Software, San Diego, CA, USA) with p-values less than 0.05 indicating statistical significance.
Results
The mean age of the subjects was 61 years (range: 27-84 years). Among the 79 subjects that completed the study, 47 subjects had their tumor located in the right breast (59.5%) and 32 had their tumor located in the left breast (40.5%). The majority of subjects had their tumors located in the upper-outer-quadrant area of the breast (n=57; 72.2%), 12 subjects had their tumors located in the upper-inner-quadrant (15.2%), 8 subjects had their tumors located in the lower-outer-quadrant (10.1%) and 2 subjects had their tumors located in the lower-inner-quadrant (2.5%). The mean tumor size at the time of the diagnosis was 1.8 cm with a range of 0.3 to 9.7 cm.
Table 1 shows a summary of the subjects’ demographics and tumor location.
Table 1:
Study demographics
| Subjects | |
| n= 79 | |
| Gender | |
| • Female | n= 79 (100%) |
| Age | |
| • Mean: 61 years (range: 27 - 84 years) | |
| Side | |
| • Right | n= 47 (59.5%) |
| • Left | n= 32 (40.5%) |
| Location | |
| • Upper outer quadrant | n= 57 (72.2%) |
| • Upper inner quadrant | n= 12 (15.2%) |
| • Lower outer quadrant | n= 8 (10.1%) |
| • Lower outer quadrant | n= 2 (2.5%) |
| Tumor size | |
| • Mean: 1.8 cm (range: 0.3 - 9.7 cm) | |
| Neoadjuvant chemotherapy | |
| • Yes | n= 18 (22.8%) |
| • No | n= 61 (77.2%) |
Subjects that underwent neoadjuvant chemotherapy (NAC) prior to the surgical procedure were not excluded from the study. Thus, out of the 79 subjects, 18 underwent NAC prior to their surgery (22.8%).
At the time of diagnosis, 14 subjects had LNs that were determined to be suspicious and underwent US-guided core biopsy. Pathology results showed metastatic disease in 8 of the 14 subjects, while the remaining 6 subjects were determined to have benign lymphatic tissue only. The specific type of surgical procedure performed was determined by the clinical team as part of the subject’s standard of care. Among the 79 subjects, 48 underwent a lumpectomy (60.8%) and 31 underwent a mastectomy (39.2%).
Table 2 summarizes tumor types and receptors. The pathology results from the US-guided core biopsies that determined the presence of cancer showed that 62 subjects had invasive ductal carcinoma (78.5%), 5 subjects had ductal carcinoma in situ (6.3%), 11 subjects had invasive lobular carcinoma (13.9%) and 1 subject had a papillary carcinoma (1.3%).
Table 2:
Tumor types and receptors
| Type of surgery | |
| • Lumpectomy | n= 48 (60.8%) |
| • Mastectomy | n= 31 (39.2%) |
| Lymph node core biopsy prior to surgery | |
| • Yes | n= 14 (17.7%) |
| • No | n= 65 (82.3%) |
| Lymph node core biopsy prior to surgery pathology results | |
| • Metastatic disease | n= 8 (57.1%) |
| • Benign lymph node tissue | n= 6 (42.9%) |
| Types of tumor determined by core biopsy prior to surgery | |
| • Invasive ductal carcinoma | n= 62 (78.5%) |
| • Ductal carcinoma in situ | n= 5 (6.3%) |
| • Invasive lobular carcinoma | n= 11 (13.9%) |
| • Papillary carcinoma | n= 1 (1.3%) |
| Types of tumor determined by pathology after surgery | |
| • Invasive ductal carcinoma | n= 56 (70.9%) |
| • Ductal carcinoma in situ | n= 3 (3.8%) |
| • Invasive lobular carcinoma | n= 12 (15.2%) |
| • Papillary carcinoma | n= 1 (1.3%) |
| • No residual tumor | n= 7 (8.8%) |
| Estrogen receptor (ER) | |
| • Positive | n= 67 (84.8%) |
| • Negative | n= 12 (15.2%) |
| Progesterone receptor (ER) | |
| • Positive | n= 59 (74.7%) |
| • Negative | n= 20 (25.3%) |
| Human epidermal growth factor receptor 2 (Her2) | |
| • Positive | n= 10 (12.7%) |
| • Negative | n= 69 (87.3%) |
There was no residual cancer found in the surgical specimen of 7 subjects, all of whom had undergone NAC for their cancers between their initial biopsy and their definitive surgery. Therefore, there was a change in the final number of the types of tumor on the subjects on the study. The final pathology results showed that 56 subjects had invasive ductal carcinoma (70.9%), 3 subjects had ductal carcinoma in situ (3.8%), 12 subjects had invasive lobular carcinoma (15.2%), 1 subject had a papillary carcinoma (1.3%) and 7 subjects had no residual tumor after NAC (8.8%).
Tumor receptor analyzes showed that the majority of subjects had positive estrogen receptor (ER; n= 67, 84.8%), positive progesterone receptor (PR; n=59. 74.7%) and negative human epidermal growth factor receptor 2 (Her-2; n=69, 87.3%).
All the UCA injections were technically successful. There were only 2 subjects, one with 2 SLNs and another with 3 SLNs which were not identified in the initial CEUS examination, however all 5 SLNs were confirmed to have microbubbles inside them in the operation room after the surgical excision by the observation of Sonazoid uptake using CDI. The hypothesis for this discrepancy is that these subjects were some of the initial study subjects enrolled in the study and perhaps not enough time was given between the injection and the end of the initial CEUS examination for the microbubbles to move from the injection site to the SLNs.
CEUS examination performed prior to the surgical procedure using lymphosonography identified 217 SLNs, average of 2.7 SLNs/subject. All SLNs identified had their location and size measurements recorded, and US images were acquired for all SLNs. No AEs occurred in any subject.
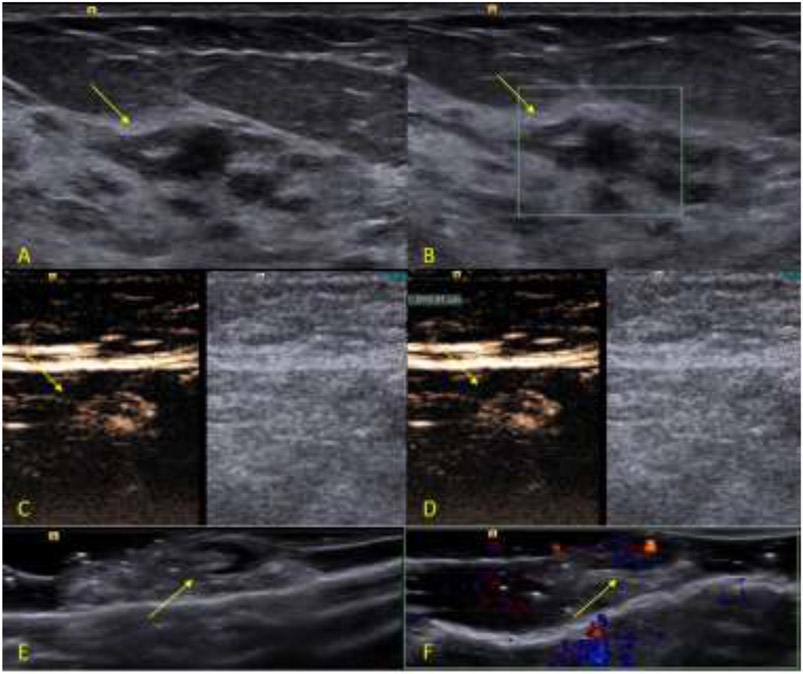
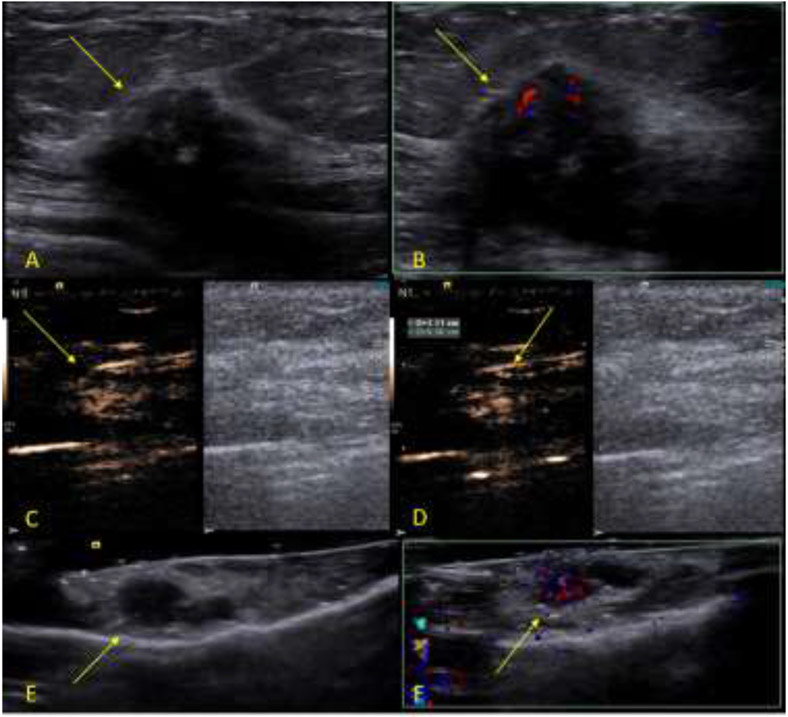
A total of 252 SLNs were surgically excised from the 79 subjects, average: 3.2 SLNs/subject, and sent to pathology to determine the final diagnosis. Thirty-four (34) SLNs from the 252 excised SLNs were determined to have metastatic disease by pathology (13.5%). These 34 SLNs were excised from 18 subjects (average 1.9 SLNs/subject). The remaining 218 SLNs excised were determined by pathology to be negative for metastatic disease. Figure 2 shows an example of a case where the SLN was determine to be benign by pathology and Figure 3 shows an example of a malignant SLN.
Figure 2:
Example of a benign study case. The subject is a 76 years-old female patient diagnosed with an invasive ductal carcinoma breast cancer located on the left breast at 2 o’clock position measuring 1.0 cm. After the surgical excision, the SLN was sent to pathology, which determine to be negative for metastatic disease. The SLN was positive for the presence of blue dye, radioactive tracer and UCA at the time of the excision. A, B-mode image of the tumor (arrow). FB, Color Doppler image of the tumor (arrow). C, Dual-image CEUS and B-mode of the SLN (arrow). D, Dual-image CEUS and B-mode of the SLN with measurement (arrow). E, B-mode image of the ex-vivo specimen of the SLN seen in C and D (arrow). F, Color Doppler image of the ex vivo specimen of the SLN seen in C and D showing the uptake of UCA (arrow).
Figure 3:
Example of a malignant study case. The subject is a 67 years-old female patient diagnosed with an invasive ductal carcinoma breast cancer located on the left breast at 2 o’clock position measuring 2.1 cm. After the surgical excision, the SLN was sent to pathology, which determine to be positive for metastatic disease. The SLN was positive for the presence of blue dye, radioactive tracer and UCA at the time of the excision. A B-mode image of the tumor (arrow). B, Color Doppler image of the tumor (arrow). C, Dual-image CEUS and B-mode of the SLN (arrow). D, Dual-image CEUS and B-mode of the SLN with measurement (arrow). E, B-mode image of the ex-vivo specimen of the SLN seen in C and D (arrow). F, Color Doppler image of the ex-vivo specimen of the SLN seen in C and D showing the uptake of UCA (arrow).
All 252 SLNs were classified as positive or negative for the presence of the 3 lymphatic tracers used in the study: blue dye, radioactive tracer and UCA. The number of excised SLNs positive by lymphatic tracers were 158 SLNs for blue dye (62.7%), 222 SLNs for radioactive tracer (88.1%) and 223 SLNs for UCA (88.5%).
The lymphatic tracers were compared and relative to blue dye both the radioactive tracer and the UCA results showed significant statistical differences (p<0.0001), while the comparison between the radioactive tracer and the UCA showed no significant difference (p>0.50). When using the blue dye positive results as the standard, the accuracy of both radioactive tracer (96.2%) and UCA (99.4%) showed no significant statistical difference (p>0.15). The comparison using the radioactive tracer as the standard, produced a significantly lower accuracy from blue dye than from lymphosonography (68.5% vs 86.5%; p<0.0001).
Pathology results showed that 34 SLNs of the 252 SLNs excised were positive for metastatic disease. Of the 34 SLNs, 18 were positive for blue dye translating to detection rate of 53%, 23 were positive for radioactive tracer translating to accuracy detection rate of 68% and 34 were positive for UCA equivalent to accuracy detection rate 100% (p<0.0001). The pathology results for malignancy included in the data analysis were not separated between macro- and micrometastasis. Any pathological findings of cancer cells inside the SLN determined the SLN as malignant.
Table 3 shows a summary of the findings.
Table 3:
Study results on nodal burden
| Subjects nodal burden | |
| • Total subjects studied | n= 79 |
| • Positive for metastatic disease | n= 18 (22.8%) |
| • Negative for metastatic disease | n= 61 (77.2%) |
| Lymph node nodal burden | |
| • Total sentinel lymph nodes surgically excised | n= 252 |
| • Positive for metastatic disease | n= 34 (13.5%) |
| • Negative for metastatic disease | n= 218 (86.5%) |
| Lymphosonography nodal burden | |
| • Total sentinel lymph nodes | n= 217 |
| • Positive for metastatic disease | n= 34 (15.7%) |
| • Negative for metastatic disease | n= 183 (84.3%) |
| Blue dye nodal burden | |
| • Total sentinel lymph nodes | n= 158 |
| • Positive for metastatic disease | n= 18 (11.4%) |
| • Negative for metastatic disease | n= 140 (88.6%) |
| Radioactive tracer nodal burden | |
| • Total sentinel lymph nodes | n= 222 |
| • Positive for metastatic disease | n= 23 (10.4%) |
| • Negative for metastatic disease | n= 199 (89.6%) |
| Ex vivo UCA nodal burden | |
| • Total sentinel lymph nodes | n= 223 |
| • Positive for metastatic disease | n= 34 (15.2%) |
| • Negative for metastatic disease | n= 189 (84.8%) |
| Sentinel lymph nodes positive for metastatic disease | |
| • Total sentinel lymph nodes | n= 34 |
| • Lymphosonography | n= 34 (100%) |
| • Blue dye | n= 18 (52.9%) |
| • Radioactive tracer | n= 23 (67.6%) |
| • Ex vivo UCA | n= 34 (100%) |
When comparing the number of SLNs identified with lymphosonography (n=217) to the number of excised SLNs that were positive for UCA (n=223) no significant statistical difference was observed (p>0.50). All the 34 SLNs that pathology determined to have metastatic disease were identified with in vivo lymphosonography and were positive for UCA after excision in the SLN specimens, resulting in a detection rate of 100% both in vivo and ex vivo.
The difference between the number of SLNs identified with lymphosonography and the number of SLNs, which were positive for UCA after excision most likely occurred due to the delay in the enhancement by the UCA. However, since this difference was not statistically significant it implies that there is no need to delay the lymphosonography examination. Starting the examination within 5 minutes of UCA injection and scanning up to 30 minutes afterwards not only produce reliable results, but also does not delay the patients’ clinical care.
Discussion
The determination of the presence and extent of axillary LN involvement remains the most powerful predictor of recurrence and survival of breast cancer patients and is used to guide treatment selection (Machado et al. 2018; Kim, Giuliano, and Lyman 2006; Moody et al. 2017; Shimazu et al. 2019; Liu et al. 2021; Deng et al. 2021; Lyman et al. 2005). Histopathologic tissue analysis is currently the only way to accurately determine LN involvement (Lyman et al. 2005). At the time of the cancer diagnosis, a LN considered to be suspicious clinically or radiologically will undergo core-biopsy to determine diagnosis, which is used as part of the initial staging and therefore, help established treatment. However, the majority of patients will have no suspicious LN findings at the time of diagnosis, which leaves surgical excision as the only way to determine the final stage of disease. Accurate assessment of potential LN involvement is essential to limit the removal to only the affected LNs as a way to minimize the anatomical disruption caused by an extensive axillary LN removal, which can result in lymphedema, nerve injury, shoulder dysfunction, and other complications that may compromise functionality and quality of life (Lyman et al. 2005; Jang et al. 2022).
Currently, mapping the lymphatic system is performed after the injection of blue dye and/or a radioactive tracer followed by a surgical excision (Emerson et al. 2012; Hill et al. 1999; Eshima et al. 2000; Veronesi et al. 2003). This approach has several limitations. The use of radioactive tracers can give an indication of the position of the SLN through the use of a hand held radiation detection probe (without any imaging component), but with the additional use of radiation (Hill et al. 1999; Goldfarb et al. 1998; Goldberg et al. 2005; Goldberg et al. 2011). Moreover, by the time of the surgical excision, the small size of the radioactive colloid means that the isotope may have passed through the SLN entering secondary and/or tertiary LNs, causing unnecessary resection of LNs (Goldberg et al. 2005; Goldberg et al. 2011; Hill et al. 1999; Goldfarb et al. 1998). The same issues are seen with the injection of blue dye, where the velocity at which the blue dye proceeds determine that the injection of blue dye has to be done at the beginning of the surgical excision (Goldfarb et al. 1998; Hill et al. 1999; Goldberg et al. 2005; Goldberg et al. 2011).
The development of the lymphosonography technique, which uses CEUS to evaluate LN, addressed the limitations of the currently used lymphatic mapping techniques. Our pre-clinical studies using a Sinclair swine model with naturally occurring melanoma tumors showed that the UCA used as lymphatic tracer in lymphosonography stay restricted to the SLNs and does not move forward in the lymphatic system (Goldberg et al. 2005; Goldberg et al. 2011; Goldberg et al. 2004; Liu et al. 2014). The translation to clinical trials with human subjects was successful with studies focused on the use of this technique to evaluate the presence of SLNs in humans with breast cancer, where the UCA was injected subdermally around the areola and the LCs followed to the axilla for the identification of SLNs with accuracies for SLN detection varying from 70 to 100% (Sever et al. 2011; Sever, Mills, Weeks, et al. 2012; Sever, Mills, Jones, et al.2012; Omoto et al. 2009; Deng et al. 2021; Moody et al. 2017; Shimazu et al. 2019; Liu et al. 2021; Cox et al. 2018; Cox et al. 2016; Cox et al. 2013).
This study represents our group’s translation of lymphosonography into a clinical trial where the technique can be evaluated in patients with breast cancer undergoing surgical excision. The major difference in our approach is that the subcutaneous injection of Sonazoid was done around the tumor area in order to identify the precise lymphatic region connected with the tumor Sonazoid is a very stable as well as reticulo-endothelial system specific UCA, which ensures that the microbubbles are eliminated by the macrophages and therefore becomes an intracellular UCA for a period of time [around 24h; (Goldberg et al. 2005; Machado et al. 2018)]. The SLN enhancement will last just as long. As part of the standard of care at our institution, both blue dye and radioactive tracer are injected periareolarly, while the UCA in our research study was injected around the tumor. The results from our study seem to indicate that injecting the lymphatic tracer around the tumor is a more node specific approach, however, there was no direct comparison performed (e.g., using the same type of lymphatic tracer in both locations). Hence, this study cannot confirm this hypothesis, which will require future research.
A total of 252 SLNs were surgically excised guided by the standard of care lymphatic tracers: blue dye and radioactive tracer. The number of positive SLNs by the lymphatic tracers were 158 SLNs for blue dye (62.7%), 222 SLNs for radioactive tracer (88.1%) and 223 SLNs for UCA (88.5%’ cf., Table 3). These results corroborate the similar accuracy seen between standard of care lymphatic tracers and CEUS in previous studies (Liu et al. 2021; Sever et al. 2011; Sever, Mills, Weeks, et al. 2012; Sever, Mills, Jones, et al. 2012; Moody et al. 2017; Shimazu et al. 2019; Deng et al. 2021; Omoto et al. 2009). The difference between the number of SLNs excised and the number positive by the standard of care lymphatic tracers indicates that without an imaging component they can guide the surgeon to a location but not to a specific SLN.
Pathology showed that 34 of the 252 excised SLNs were positive for metastatic disease out of which 18 were positive for blue dye (detection rate: 53%), 23 were positive for radioactive tracer (detection rate: 68%) and 34 were positive for lymphosonography (detection rate: 100%). These results validate our group’s choice to inject the UCA around the tumor area instead of around the areola, since finding all the SLNs with metastatic disease is vital for patient care.
However, this study objective was SLN identification, therefore once microbubbles were seen in the SLN it was considered identified and we did not wait for microbubbles saturation inside the SLN in order to determine any potential signs of malignancy. Future studies will be required to both identify and characterize the SLNs.
Conclusion
In conclusion, this study demonstrated that lymphosonography achieved similar accuracy as the standard of care methods for identifying SLNs in breast cancer patients, with the added advantage of an imaging component that allows for a pre-operative evaluation of SLNs. All the 34 malignant SLNs were identified by lymphosonography indicating that this may be a more specific and precise approach to SLN identification in patients with breast cancer.
Acknowledgements
This work was supported by NIH grant R01 CA172336.
Sonazoid was supplied by GE Healthcare, Oslo, Norway.
The S3000 HELX scanner was provided by Siemens Healthineers, Mountain View, CA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data generated or analyzed during the study are available from the corresponding author by request.
References:
- Boughey Judy C., Suman Vera J., Mittendorf Elizabeth A., Ahrendt Gretchen M., Wilke Lee G., Taback Bret, Leitch A. Marilyn, Flippo-Morton Teresa S., Kuerer Henry M., and Bowling Monet. 2015. 'Factors affecting sentinel lymph node identification rate after neoadjuvant chemotherapy for breast cancer patients enrolled in ACOSOG Z1071 (Alliance)', Annals of surgery, 261: 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabioğlu Neslihan, Karanlik Hasan, Kangal Dilek, Özkurt Enver, Öner Gizem, Sezen Fatma, Yilmaz Ravza, Tükenmez Mustafa, Önder Semen, and İğci Abdullah. 2018. 'Improved false-negative rates with intraoperative identification of clipped nodes in patients undergoing sentinel lymph node biopsy after neoadjuvant chemotherapy', Annals of Surgical Oncology, 25: 3030–36 [DOI] [PubMed] [Google Scholar]
- Camp ER, Cendan JC, Feezor R, and Lind DS. 2004. 'The hottest sentinel lymph node is not always the positive node', The American surgeon, 70: 475. [PubMed] [Google Scholar]
- Cho Nariya, Moon Woo Kyung, Han Wonshik, Park In Ae, Cho Jihyoung, and Noh Dong-Young. 2009. 'Preoperative sonographic classification of axillary lymph nodes in patients with breast cancer: node-to-node correlation with surgical histology and sentinel node biopsy results', American Journal of Roentgenology, 193: 1731–37 [DOI] [PubMed] [Google Scholar]
- Cox K, Sever A, Jones S, Weeks J, Mills P, Devalia H, Fish D, and Jones P. 2013. 'Validation of a technique using microbubbles and contrast enhanced ultrasound (CEUS) to biopsy sentinel lymph nodes (SLN) in pre-operative breast cancer patients with a normal grey-scale axillary ultrasound', European Journal of Surgical Oncology (EJSO), 39: 760–65 [DOI] [PubMed] [Google Scholar]
- Cox Karina, Taylor-Phillips Sian, Sharma Nisha, Weeks Jennifer, Mills Philippa, Sever Ali, Lim Adrian, Haigh Isobel, Hashem Mohamed, and De Silva Tania. 2018. 'Enhanced pre-operative axillary staging using intradermal microbubbles and contrast-enhanced ultrasound to detect and biopsy sentinel lymph nodes in breast cancer: a potential replacement for axillary surgery’, The British Journal of Radiology, 91: 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox Karina, Weeks Jennifer, Mills Pippa, Chalmers Ritchie, Devalia Haresh, Fish David, and Sever Ali. 2016. 'Contrast-enhanced ultrasound biopsy of sentinel lymph nodes in patients with breast cancer: implications for axillary metastases and conservation', Annals of Surgical Oncology, 23: 58–64 [DOI] [PubMed] [Google Scholar]
- Deng Huadong, Lei Jianming, Jin Lixian, and Shi Hongwei. 2021. 'Diagnostic efficacy of sentinel lymph node in breast cancer under percutaneous contrast-enhanced ultrasound: An updated metaanalysis', Thoracic Cancer: 1759–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson Derek K., Limmer Karl K., Hall David J., Han Sung-Ho, Eckelman William C., Kane Christopher J., Wallace Anne M., and Vera David R.. 2012. A receptor-targeted fluorescent radiopharmaceutical for multireporter sentinel lymph node imaging', Radiology, 265: 186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshima Sennis, Fauconnier Theresa, Eshima Lorie, and Thornback John R.. 2000. "Radiopharmaceuticals for Lymphoscintigraphy: Including dusimetry and radiation considerations." In, 25–32 Elsevier. [DOI] [PubMed] [Google Scholar]
- Evans Kevin D., and Boyd Ashley. 2007. 'Sonographic and vascular assessment of axillary lymph nodes: a review Journal of diagnostic medical sonography, 23: 63–72 [Google Scholar]
- Forsberg F, Merton DA, Liu JB, Needleman L, and Goldberg BB. 1998. 'Clinical applications of ultrasound contrast agents', Ultrasonics, 36: 695–701 [DOI] [PubMed] [Google Scholar]
- Forsberg Flemming, Piccoli Catherine W., Liu Ji-Bin, Rawool Nandkumar M., Merton Daniel A., Mitchell Donald G., and Goldberg Barry B.. 2002. 'Hepatic tumor detection: MR imaging and conventional US versus pulse-inversion harmonic US of NC100100 during its reticuloendothelial system–specific phase', Radiology, 222: 824–29 [DOI] [PubMed] [Google Scholar]
- Forsberg Flemming, Shi William T., and Goldberg BB. 2000. 'Subharmonic imaging of contrast agents', Ultrasonics, 38: 93–98 [DOI] [PubMed] [Google Scholar]
- Goldberg Barry B., Liu Ji-Bin, and Forsberg Flemming. 1994. 'Ultrasound contrast agents: a review', Ultrasound in medicine & biology, 20: 319–33 [DOI] [PubMed] [Google Scholar]
- Goldberg Barry B., Merton Daniel A., Liu Ji-Bin, Forsberg Flemming, Zhang Kaijun, Thakur Madhukar, Schulz Stephanie, Schanche Robin, Murphy George F., and Waldman Scott A.. 2011. 'Contrast-enhanced ultrasound imaging of sentinel lymph nodes after peritumoral administration of Sonazoid in a melanoma tumor animal model', Journal of Ultrasound in Medicine, 30: 441–53 [DOI] [PubMed] [Google Scholar]
- Goldberg Barry B., Merton Daniel A., Liu Ji-Bin, Murphy George, and Forsberg Flemming. 2005. 'Contrast-enhanced sonographic imaging of lymphatic channels and sentinel lymph nodes', Journal of Ultrasound in Medicine, 24: 953–65 [DOI] [PubMed] [Google Scholar]
- Goldberg Barry B., Merton Daniel A., Liu Ji-Bin, Thakur Mathew, Murphy George F., Needleman Larry, Tornes Audun, and Forsberg Flemming. 2004. 'Sentinel lymph nodes in a swine model with melanoma: contrast-enhanced lymphatic US', Radiology, 230: 727–34 [DOI] [PubMed] [Google Scholar]
- Goldfarb Leonard R., Alazraki Naomi P., Eshima Dennis, Eshima Lorie A., Herda Stephen C., and Halkar Raghuveer K.. 1998. 'Lymphoscintigraphic identification of sentinel lymph nodes: clinical evaluation of 0.22-micron filtration of Tc-99m sulfur colloid', Radiology, 208: 505–09 [DOI] [PubMed] [Google Scholar]
- Govaert GAM, Oostenbroek RJ, and Plaisier PW. 2005. 'Prolonged skin staining after intradermal use of patent blue in sentinel lymph node biopsy for breast cancer', European Journal of Surgical Oncology (EJSO), 31: 373–75 [DOI] [PubMed] [Google Scholar]
- Hill Arnold D. K., Mann Bruce G., Borgen Patrick I., and Cody Hiram S. Iii. 1999. 'Sentinel lymphatic mapping in breast cancer', Journal of the American College of Surgeons, 188: 545–49 [DOI] [PubMed] [Google Scholar]
- Jang Samuel, Lee Christine U., Hesley Gina K., Knudsen John M., Brinkman Nathan J., and Tran Nho V.. 2022. 'Lymphatic Mapping Using US Microbubbles before Lymphaticovenous Anastomosis Surgery for Lymphedema', Radiology: 21–23. [DOI] [PubMed] [Google Scholar]
- Jung So-Youn, Han Jai Hong, Park Soo Jin, Lee Eun-Gyeong, Kwak Joohwa, Kim Sun Hye, Lee Moo Hyun, Lee Eun Sook, Kang Han-Sung, and Lee Keun Seok. 2019. 'The sentinel lymph node biopsy using indocyanine green fluorescence plus radioisotope method compared with the radioisotope-only method for breast cancer patients after neoadjuvant chemotherapy: a prospective, randomized, open-label, single-center phase 2 trial', Annals of Surgical Oncology, 26: 2409–16 [DOI] [PubMed] [Google Scholar]
- Kang Seok Hyung, Kim Seok-Ki, Kwon Youngmee, Kang Han-Sung, Kang Jae Hee, Ro Jungsil, and Lee Eun Sook. 2004. 'Decreased identification rate of sentinel lymph node after neoadjuvant chemotherapy', World journal of surgery, 28: 1019–24 [DOI] [PubMed] [Google Scholar]
- Kim Theodore, Giuliano Armando E., and Lyman Gary H.. 2006. 'Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis', Cancer, 106: 4–16 [DOI] [PubMed] [Google Scholar]
- King Tari A., Fey Jane V., Van Zee Kimberly J., Heerdt Alexandra S., Gemignani Mary L., Port Elisa Rush, Sclafani Lisa, Sacchini Virgilio, Petrek Jeanne A., and Cody Hiram S.. 2004. A prospective analysis of the effect of blue-dye volume on sentinel lymph node mapping success and incidence of allergic reaction in patients with breast cancer', Annals of Surgical Oncology, 11: 535–41 [DOI] [PubMed] [Google Scholar]
- Kinoshita Takayuki, Takasugi Miyuki, Iwamoto Eriko, Akashi-Tanaka Sadako, Fukutomi Takashi, and Terui Shoji. 2006. 'Sentinel lymph node biopsy examination for breast cancer patients with clinically negative axillary lymph nodes after neoadjuvant chemotherapy', The American journal of surgery, 191: 225–29 [DOI] [PubMed] [Google Scholar]
- Liu Ji-Bin, Merton Daniel A., Berger Adam C., Forsberg Flemming, Witkiewicz Agnieszka, Zhao Hongjia, Eisenbrey John R., Fox Traci B., and Goldberg Barry B.. 2014. 'Contrast-enhanced sonography for detection of secondary lymph nodes in a melanoma tumor animal model', Journal of Ultrasound in Medicine, 33: 939–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Yan-Bing, Xia Mei, Li Yun-Jie, Li Sheng, Li Hao, and Li Yun-Ling. 2021. 'Contrast-Enhanced Ultrasound in Locating Axillary Sentinel Lymph Nodes in Patients with Breast Cancer: A Prospective Study', Ultrasound in medicine & biology, 47: 1475–83 [DOI] [PubMed] [Google Scholar]
- Lyman Gary H., Giuliano Armando E., Somerfield Mark R., Benson Al B., Bodurka Diane C., Burstein Harold J., Cochran Alistair J., Cody Hiram S., Edge Stephen B., and Galper Sharon. 2005. 'American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer', Journal of Clinical Oncology, 23: 7703–20. [DOI] [PubMed] [Google Scholar]
- Lyshchik Andrej. 2019. Specialty Imaging: Fundamentals ofCEUS E-Book (Elsevier Health Sciences; ). [Google Scholar]
- Machado Priscilla, Stanczak Maria, Liu Ji-Bin, Moore Jason N., Eisenbrey John R., Needleman Laurence, Kraft Walter K., and Forsberg Flemming. 2018. 'Subdermal ultrasound contrast agent injection for sentinel lymph node identification: an analysis of safety and contrast agent dose in healthy volunteers', Journal of Ultrasound in Medicine, 37: 1611–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattrey Robert F., Kono Yuko, Baker Kris, and Peterson Tom. 2002. 'Sentinel lymph node imaging with microbubble ultrasound contrast material', Academic radiology, 9: 231–35. [DOI] [PubMed] [Google Scholar]
- Moody A. Nielsen, Bull J, Culpan AM, Munyombwe T, Sharma Nisha, Whitaker M, and Wolstenhulme S. 2017. 'Preoperative sentinel lymph node identification, biopsy and localisation using contrast enhanced ultrasound (CEUS) in patients with breast cancer: a systematic review and meta-analysis', Clinical radiology, 72: 959–71. [DOI] [PubMed] [Google Scholar]
- Omoto Kiyoka, Matsunaga Hiroaki, Take Natsuki, Hozumi Yasuo, Takehara Megumi, Omoto Yawara, Shiozawa Mikio, Mizunuma Hirobumi, Harashima Hiroki, and Taniguchi Nobuyuki. 2009. 'Sentinel node detection method using contrast-enhanced ultrasonography with sonazoid in breast cancer: preliminary clinical study', Ultrasound in medicine & biology, 35: 1249–56. [DOI] [PubMed] [Google Scholar]
- Romeo Valeria, Accardo Giuseppe, Perillo Teresa, Basso Luca, Garbino Nunzia, Nicolai Emanuele, Maurea Simone, and Salvatore Marco. 2021. 'Assessment and prediction of response to neoadjuvant chemotherapy in breast cancer: A comparison of imaging modalities and future perspectives', Cancers, 13: 3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sever AR, Mills P, Jones SE, Mali W, and Jones PA. 2012. 'Sentinel node identification using microbubbles and contrast-enhanced ultrasonography', Clinical radiology, 67: 687–94 [DOI] [PubMed] [Google Scholar]
- Sever Ali R., Mills Philippa, Jones Susan E., Cox Karina, Weeks Jennifer, Fish David, and Jones Peter A.. 2011. 'Preoperative sentinel node identification with ultrasound using microbubbles in patients with breast cancer', American Journal of Roentgenology, 196: 251–56 [DOI] [PubMed] [Google Scholar]
- Sever Ali R., Mills Philippa, Weeks Jennifer, Jones Susan E., Fish David, Jones Peter A., and Mali Willem. 2012. 'Preoperative needle biopsy of sentinel lymph nodes using intradermal microbubbles and contrast-enhanced ultrasound in patients with breast cancer', American Journal of Roentgenology, 199: 465–70 [DOI] [PubMed] [Google Scholar]
- Shimazu Kenzo, Miyake Tomohiro, Tanei Tomonori, Naoi Yasuto, Shimoda Masafumi, Kagara Naofumi, Kim Seung Jin, and Noguchi Shinzaburo. 2019. 'Real-time visualization of lymphatic flow to sentinel lymph nodes by contrast-enhanced ultrasonography with sonazoid in patients with breast cancer', Ultrasound in medicine & biology, 45: 2634–40 [DOI] [PubMed] [Google Scholar]
- Tan Veronique K. M., Goh Brian K. P., Fook-Chong Stephanie, Khin Lay-Wai, Wong Wai-Keong, and Yong Wei-Sean. 2011. 'The feasibility and accuracy of sentinel lymph node biopsy in clinically node-negative patients after neoadjuvant chemotherapy for breast cancer—a systematic review and meta-analysis', Journal of surgical oncology, 104: 97–103 [DOI] [PubMed] [Google Scholar]
- Tokin Christopher A., Cope Frederick O., Metz Wendy L., Blue Michael S., Potter Beth M., Abbruzzese Bonnie C., Flartman Richard D., Joy Marcus T., King Dennis W., and Christman Lori A.. 2012. 'The efficacy of Tilmanocept in sentinel lymph mode mapping and identification in breast cancer patients: a comparative review and meta-analysis of the 99mTc-labeled nanocolloid human serum albumin standard of care', Clinical & experimental metastasis, 29: 681–86 [DOI] [PubMed] [Google Scholar]
- Veronesi Umberto, Paganelli Giovanni, Viale Giuseppe, Luini Alberto, Zurrida Stefano, Galimberti Viviana, Intra Mattia, Veronesi Paolo, Robertson Chris, and Maisonneuve Patrick. 2003. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer', New England Journal of Medicine, 349: 546–53 [DOI] [PubMed] [Google Scholar]
- Voit Christiane A., van Akkooi Alexander C. J., Schafer-Hesterberg Gregor, Schoengen Alfred, Schmitz Paul I. M., Sterry Wolfram, and Eggermont Alexander M. M.. 2009. 'Rotterdam Criteria for sentinel node (SN) tumor burden and the accuracy of ultrasound (US)-guided fine-needle aspiration cytology (FNAC): can US-guided FNAC replace SN staging in patients with melanoma?', Journal of Clinical Oncology, 27: 4994–5000 [DOI] [PubMed] [Google Scholar]
- Wohrl S, Focke M, Hinterhuber G, Stingl G, and Binder M. 2004. 'Near-fatal anaphylaxis to patent blue V', Journal of Allergy and Clinical Immunology, 113: 72. [DOI] [PubMed] [Google Scholar]