Figure 2.
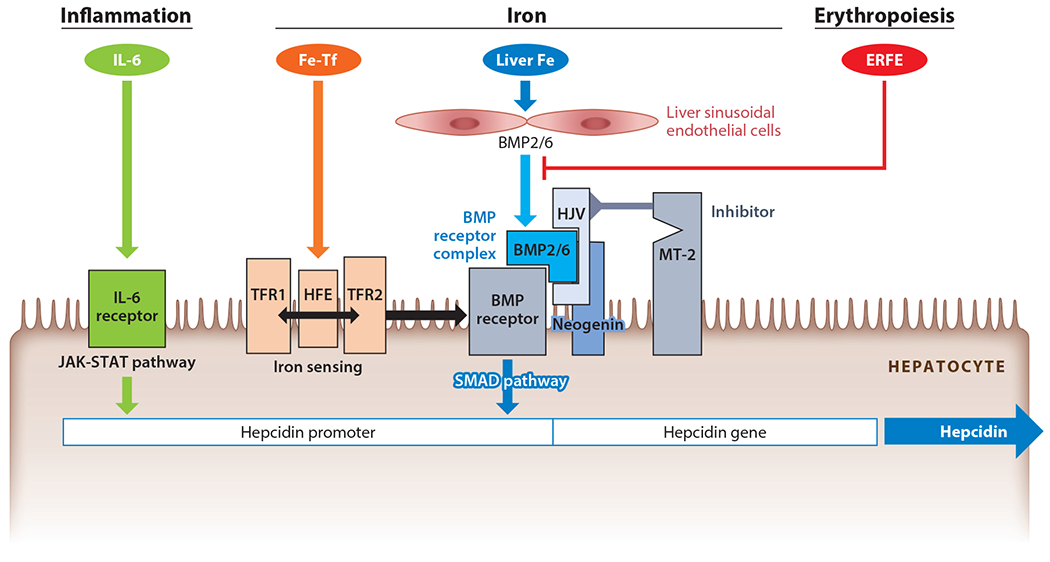
Molecular mechanisms of hepcidin regulation. Center: Iron regulates hepcidin through two distinct mechanisms. Extracellular iron in the form of holotransferrin (Fe-Tf) is sensed by the two transferrin receptors (TFR1 and TFR2). Binding of Fe-Tf to its receptors promotes hemochromatosis protein (HFE) interaction with TFR2 instead of TFR1, and the HFE/TFR2 complex then sensitizes the bone morphogenetic protein (BMP) receptor to its ligands BMP2 and −6 or the BMP2/6 heterodimer. HFE may also directly stabilize the BMP receptor by preventing its ubiquitination. Hemojuvelin (HJV), a membrane-linked BMP coreceptor, potentiates the BMP receptor activation. Once activated, BMP receptors initiate SMAD signaling, which increases hepcidin transcription. Increased intracellular iron in the liver enhances BMP6 and −2 production by liver sinusoidal endothelial cells, eventually leading to activation of the BMP receptor on hepatocytes. Under low-iron conditions, low Fe-Tf concentrations and low intracellular iron both lead to decreased BMP pathway signaling and decreased hepcidin mRNA expression. Furthermore, matriptase-2 (MT-2) protease is stabilized in low-iron conditions and inhibits and cleaves HJV and other molecules of the BMP pathway, thus further decreasing the SMAD signaling. Left: Inflammation stimulates hepcidin production by increasing the transcription of hepcidin through the interleukin (IL)-6–JAK-STAT pathway. Right: Erythropoiesis activation leads to increased production of erythroferrone (ERFE) by erythroblasts. ERFE then inhibits BMP signaling by binding to BMP2/6 and interfering with its interaction with the BMP receptor, thereby lowering hepcidin and making more iron available for erythropoiesis.
