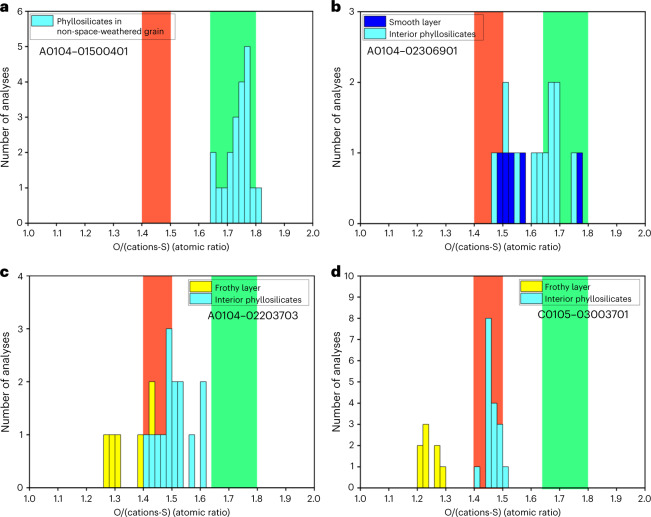
Fig. 4. Histograms of atomic ratios of oxygen to the cations bonded to oxygen in phyllosilicates, a smooth layer and frothy layers.
A mixture of saponite without interlayer H2O molecules and serpentine has a range of ratios represented by green bands. If a mixture of saponite and serpentine is decomposed into an anhydrous compound, it has a range of ratios represented by red bands. In order to calculate the atomic ratios of oxygens to the cations bonded to oxygen in phyllosilicates, we subtracted the cations bonded to sulfur (S), which were calculated based on the assumption that the ratio of the S-bonded Fe and Ni ions to S is unity for simplicity. a, Phyllosilicates in a non-space-weathered grain contain almost no interlayer H2O but preserve structural –OH groups. b, A smooth layer lost a considerable amount of structural –OH groups and phyllosilicates just below the smooth layers partially lost structural –OH groups. c, Phyllosilicates just below the frothy layer have lost the structural –OH groups considerably. d, Phyllosilicates just below the frothy layer have lost almost all the structural –OH groups.Because the frothy layers have even lower ratios than the red bands, they are also anhydrous. Their very low ratios may be related to their high abundance of embedded Fe-Ni sulfide. The ratio at the right end of the green belts is 1.8, which is calculated from the generalized chemical formula of serpentine Y6Z4O10(OH)8. O/(Y + Z) = 18/10 = 1.8. The ratio at the left end of the green belts is 1.64, which is calculated from the generalized chemical formula of saponite with no interlayer H2O molecules X0.6Y6Z8O20(OH)4. O/(X + Y + Z) = 24/14.6 = 1.64. The ratio at the right end of the red belts is 1.5, which is calculated from the generalized chemical formula of the dehydrated decomposition product of saponite X0.6Y6Z8O22. O/(X + Y + Z) = 22/14.6 = 1.5. The ratio at the left end of the red belts is 1.4, which is calculated from the generalized chemical formula of the dehydrated decomposition product of serpentine Y6Z4O14. O/(Y + Z) = 14/10 = 1.4.