Abstract
Purpose
This study aimed to clarify the characteristics of and evaluate the risk factors for radiation pneumonitis (RP) induced by chemoradiation therapy (CRT) using accelerated hyperfractionated (AHF) radiation therapy (RT) in patients with limited-stage small cell lung cancer (LS-SCLC).
Methods and Materials
Between September 2002 and February 2018, 125 patients with LS-SCLC were treated with early concurrent CRT using AHF-RT. Chemotherapy was comprised of carboplatin/cisplatin with etoposide. RT was administered twice daily (45 Gy/30 fractions). We collected data regarding onset and treatment outcomes for RP, and analyzed the relationship between RP and total lung dose–volume histogram findings. Uni- and multivariate analyses were performed to assess patient- and treatment-related factors for grade ≥2 RP.
Results
The median age of patients was 65 years, and 73.6% of participants were men. In addition, 20% and 80.0% of participants presented with disease stage II and III, respectively. The median follow-up time was 73.1 months. Grades 1, 2, and 3 RP were observed in 69, 17, and 12 patients, respectively. Grades 4 to 5 RP were not observed. RP was treated with corticosteroids in patients with grade ≥2 RP, without recurrence. The median time from initiation of RT to onset of RP was 147 days. Three patients developed RP within 59 days, 6 within 60 to 89 days, 16 within 90 to 119 days, 29 within 120 to 149 days, 24 within 150 to 179 days, and 20 within ≥180 days. Among the dose–volume histogram parameters, the percentage of lung volume receiving >30 Gy (V30) was most strongly related to the incidence of grade ≥2 RP, and the optimal threshold to predict RP incidence was V30 ≥20%. On multivariate analysis, V30 ≥20% was an independent risk factor for grade ≥2 RP.
Conclusions
The incidence of grade ≥2 RP correlated strongly with a V30 of ≥20%. Contrarily, the onset of RP induced by concurrent CRT using AHF-RT may occur later. RP is manageable in patients with LS-SCLC.
Introduction
Small cell lung cancer (SCLC) is one of the most aggressive tumor types. Nevertheless, limited-stage (LS) SCLC is curable with a combination of radiation therapy (RT) and chemotherapy.1,2 Concurrent chemoradiation therapy (CRT) using accelerated hyperfractionated (AHF) RT is the standard treatment for patients with LS-SCLC.3,4 In a previous study of patients with LS-SCLC treated with CRT, the reported incidence of radiation pneumonitis (RP) of any grade, as well as RP grade ≥2, was 83.0% to 100% and 18.6% to 25.8%, respectively.5,6
RP is one of the most common dose-limiting adverse events in thoracic RT.5,7 In patients with locally advanced (LA) non-small cell lung cancer (NSCLC) treated with CRT, several factors, including the percentage of lung volume receiving >20 Gy (V20), >30 Gy (V30), and the mean lung dose (MLD; as derived from dose–volume histograms [DVHs]), have recently been reported as indicators for the occurrence of RP.8, 9, 10, 11, 12, 13, 14, 15 Among the DVH parameters, V20 correlated well with the incidence of symptomatic RP in previous study populations.8, 9, 10, 11,13,14,16,17 Meanwhile, age, diabetes mellitus (as a comorbidity), and smoking status have been reported as the clinical factors most strongly associated with symptomatic RP in patients with LA-NSCLC.17, 18, 19, 20, 21
The risk factors for RP have not yet been thoroughly investigated in patients with LS-SCLC treated with CRT using AHF-RT. To our knowledge, no reports have focused on the treatment and outcomes for RP in this population. Therefore, the aim of this retrospective study was to clarify the characteristics of RP and evaluate the risk factors for RP, including DVH parameters, in patients with LS-SCLC treated with concurrent CRT using AHF-RT.
Methods and Materials
This retrospective study was approved by the institutional review board at our institution, and this work was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained in the form of an opt-out via the official institutional website.
Patient characteristics
We retrospectively evaluated patients with LS-SCLC who presented at our institute between September 2002 and February 2018. Patients with pathologically diagnosed SCLC, or those with combined SCLC who underwent concurrent CRT using AHF-RT, were eligible for study inclusion. Meanwhile, patients treated with late concurrent CRT (where RT was initiated after starting the third course of chemotherapy) or sequential CRT were excluded.
LS-SCLC was defined as a disease with lesions limited to the ipsilateral thorax, contralateral mediastinum, and contralateral supraclavicular fossa lymph nodes, without malignant pleural effusion or pericardial effusion. Lesion distribution was assessed by computed tomography (CT) from the chest to the abdomen or by positron emission tomography (PET). The absence of brain metastases was confirmed by magnetic resonance imaging.
For the statistical analysis, we collected data on age, sex, Eastern Cooperative Oncology Group performance status score at the time of diagnosis, baseline forced expiratory volume in 1 second/forced vital capacity percentage, smoking status, pack-years of smoking, history of diabetes mellitus, performance of PET-CT at the time of diagnosis, clinical stage at the time of diagnosis, chemotherapy regimen (cisplatin/carboplatin + etoposide [ETP]), reason for discontinuation of chemotherapy, initial date of RT, last date of RT, total lung DVHs (V5-50), maximum lung dose (Dmax), MLD, RP grade, date of RP diagnosis, initial treatment of RP, and outcomes of RP. The follow-up period was calculated from the start of RT.
RP was diagnosed by confirming the relationship between the infiltration shadow detected on CT and the distribution of the RT field, with evaluations conducted by one radiologist and one pulmonologist. The severity of RP was evaluated as the worst grade of pneumonitis according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0,22 which sets the following criteria for grade 2 RP: Symptomatic, medical intervention indicated, limiting instrumental activities of daily living.
Chemotherapy and radiation therapy
All patients received chemotherapy with platinum-based regimens: Cisplatin (60 or 80 mg/m2 on day 1) and ETP (80 or 100 mg/m2 on days 1-3), or carboplatin (area under the curve [AUC] = 5 on day 1) and ETP (80 mg/m2 on days 1-3). These regimens were repeated for up to 4 courses. Radiation treatment planning was based on slow CT scans acquired while breathing freely in the treatment position. CT data sets were transferred into a commercial treatment planning system (Pinnacle,3 Philips Medical Systems, Andover, MA). The treatment course was planned by a board-certified radiation oncologist.
Gross tumor volume (GTV) was defined as the total volume of the primary tumor and the metastatic lymph nodes, with a short-axis diameter of ≥1 cm on CT scanning and nodes of <1 cm on CT with high fluorodeoxyglucose uptake on PET scanning. The clinical target volume (CTV1) was defined as the GTV plus a uniform 5-mm margin and elective nodal regions. The second clinical target volume (CTV2) was defined as CTV1 minus elective nodal regions. Elective nodal regions included the affected lymph node stations, subcarinal region, and ipsilateral hilum, and supraclavicular lymph nodes were included in elective nodal regions if metastasis was detected. Lymph nodes with pathologic confirmation by endobronchial ultrasound-transbronchial needle aspiration, an increase in size over time, or PET/CT positivity were included in the elective nodal regions. The planning target volume was defined as the CTV1 or CTV2 plus a uniform 5-mm margin. The treatment plans were calculated with heterogeneity correction using an adaptive convolution algorithm within Pinnacle.3
RT was delivered concurrently with chemotherapy, and initiated before the start of the third course of chemotherapy, which was delivered twice daily at 1.5 Gy per fraction to a total of 45 Gy (with interfractional interval of at least 6 hours) using 3-dimensional conformal RT. The photon energy of the external beam was set to 6, 8, or 10 MV. Dose calculations were performed using tissue density inhomogeneity correction.
Treatment was delivered using a linear accelerator with a photon beam in the MV range. All treatment plans were based on volumetric CT. RT consisted of anterior–posterior opposed fields to CTV1 for the first 30 Gy of 45 Gy, followed by irradiation of off-cord oblique opposing fields to CTV2 for the next dose of 15 Gy.
Dose-volume parameters
The planned dose distributions were restored from the archived data of the treatment planning system, and confirmed to be clinically delivered through chart review. Lung V5-50 was defined as the percent volume receiving at least 5 to 50 Gy in increments of 5 Gy. Dmax was defined as the maximum dose to the total lung and MLD as the average dose to the total lung. The lung was defined as the total lung minus the GTV.
Statistical methods
Differences between the groups were compared using Fisher's exact tests for categorical variables (according to incidence of RP grade ≥2). Continuous variables were analyzed using the Wilcoxon rank-sum test. Patients were divided into 2 groups based on median values for age, pack-years of smoking, and forced expiratory volume in 1 second/forced vital capacity percentage. An analysis of V5-50, Vmax, and MLD using receiver operating characteristic (ROC) curves was used to select the most relevant threshold to predict RP grade ≥2 or 3.
A univariate logistic regression analysis was used to evaluate the association between total lung DVH parameters (V5-50, Vmax, and MLD) and RP grade ≥2. The DVH parameter with a minimal P-value and patient characteristics showing a P < .1 on univariate analysis were included in the multivariate analysis for RP grade ≥2. A multivariate analysis of risk factors related to grade ≥3 pneumonia was not performed because of the small number of patients in that category (n = 12). DVH parameters are known to be correlated with each other;5,7,15,17 thus, we included one DVH parameter, showing the maximum AUC on ROC analysis and the minimal P-value on univariate analysis in the multivariate analysis for grade ≥2 RP. To confirm this correlation, a Spearman's rank correlation analysis was used to determine correlations between DVH parameters.
The cutoff value was determined by maximizing the Youden index. All P-values were derived from 2-sided significance tests, and statistical significance was set to P < .05. Statistical analyses were performed using JMP statistical software, version 15 (SAS Institute, Cary, NC).
Results
Patient characteristics
Between September 2002 and February 2018, 173 patients with LS-SCLC were eligible for concurrent CRT at our medical center. Forty-eight patients were excluded from this study, because they were already receiving sequential RT (n = 40), late concurrent RT (n = 4), or conventional RT (n = 4). Five patients had not competed 4 courses of chemotherapy. Of the 125 patients eligible for this study, the median age was 65 years (range, 34-76 years). All enrolled patients had an Eastern Cooperative Oncology Group performance status score of 0/1, and 73.6% of patients were men; 64.0% were current smokers, 12.8% had diabetes mellitus, and 80.0% presented with disease stage III. The median pack-years of smoking was 48 years (range, 0-225 years; Table 1).
Table 1.
Patient medical and demographic characteristics
| Characteristic | All patients (N = 125) | Grade ≥2 radiation pneumonitis (n = 29) | Grade 0-1 radiation pneumonitis (n = 96) | P-value |
|---|---|---|---|---|
| Median age, years (range) | 65 (34–76) | 69 (51–75) | 65 (34–76) | .026 |
| <65 | 54 (43.2) | 8 (27.6) | 46 (47.9) | .579 |
| ≥65 | 71 (56.8) | 21 (72.4) | 50 (52.1) | |
| Sex, n (%) | ||||
| Male | 92 (73.6) | 22 (75.9) | 70 (72.9) | .815 |
| Female | 33 (26.4) | 7 (24.1) | 26 (27.1) | |
| Eastern Cooperative Oncology Group performance status score, n (%) | ||||
| 0 | 67 (53.6) | 16 (55.2) | 51 (53.1) | 1.000 |
| 1 | 58 (46.4) | 13 (44.8) | 45 (46.9) | |
| Smoking status, n (%) | ||||
| Current | 80 (64.0) | 20 (69.0) | 60 (62.5) | .175 |
| Past | 44 (35.2) | 8 (27.6) | 36 (37.5) | |
| Never | 1 (0.8) | 1 (3.5) | 0 (0.0) | |
| Pack-years of smoking | ||||
| Median (range) | 48 (0-225) | 43 (0-120) | 50 (6.3-225) | .063 |
| <48 | 61 (49.6) | 19 (65.5) | 42 (44.7) | .058 |
| ≥48 | 62 (50.4) | 10 (34.5) | 52 (55.3) | |
| Forced expiratory volume in 1 second/forced vital capacity, % | ||||
| Median (range) | 70.0 (28.6-91.3) | 69.8 (47.5-84.5) | 70.4 (28.6-91.3) | .760 |
| ≥70 | 50 (51.0) | 11 (44.0) | 39 (53.4) | .490 |
| <70 | 48 (49.0) | 14 (56.0) | 34 (46.6) | |
| Diabetes mellitus | ||||
| Yes | 16 (12.8) | 7 (24.1) | 9 (9.4) | .055 |
| No | 109 (87.2) | 22 (75.9) | 87 (90.6) | |
| Pathologic diagnosis, n (%) | ||||
| Small cell carcinoma | 119 (95.2) | 28 (96.6) | 91 (94.8) | 1.000 |
| Combined small cell lung carcinoma | 6 (4.8) | 1 (3.5) | 5 (5.2) | |
| Clinical stage (Union International for Cancer Control-TNM 8th edition), n (%) | ||||
| IIB | 25 (20.0) | 7 (24.1) | 18 (18.8) | .678 |
| IIIA | 46 (36.8) | 10 (34.5) | 36 (37.5) | |
| IIIB | 38 (30.4) | 10 (34.5) | 28 (29.2) | |
| IIIC | 16 (12.8) | 2 (6.9) | 14 (14.6) | |
| T stage, n (%) | ||||
| Tx | 3 (2.4) | 0 (0.0) | 3 (3.1) | .996 |
| T1 | 32 (25.6) | 7 (24.1) | 25 (26.0) | |
| T2 | 40 (32.0) | 10 (34.5) | 30 (31.3) | |
| T3 | 17 (13.6) | 4 (13.8) | 13 (13.5) | |
| T4 | 33 (26.4) | 8 (27.6) | 25 (26.0) | |
| N stage, n (%) | ||||
| N0 | 7 (5.6) | 1 (3.5) | 6 (6.3) | .454 |
| N1 | 29 (23.2) | 10 (34.5) | 19 (19.8) | |
| N2 | 54 (43.2) | 11 (37.9) | 43 (44.8) | |
| N3 | 35 (28.0) | 7 (24.1) | 28 (29.2) | |
| Positron emission tomography-computed tomography | ||||
| Performed | 88 (70.4) | 23 (79.3) | 65 (67.7) | .257 |
| Not performed | 37 (29.6) | 6 (20.7) | 31 (32.3) | |
Twenty-six patients developed RP grade ≥2. Despite not reaching statistical significance, trends were observed. Specifically, diabetes mellitus (P = .055) and ≥48 pack-years of smoking (P = .058) were more frequent in patients with RP grade ≥2.
Incidence of radiation pneumonitis
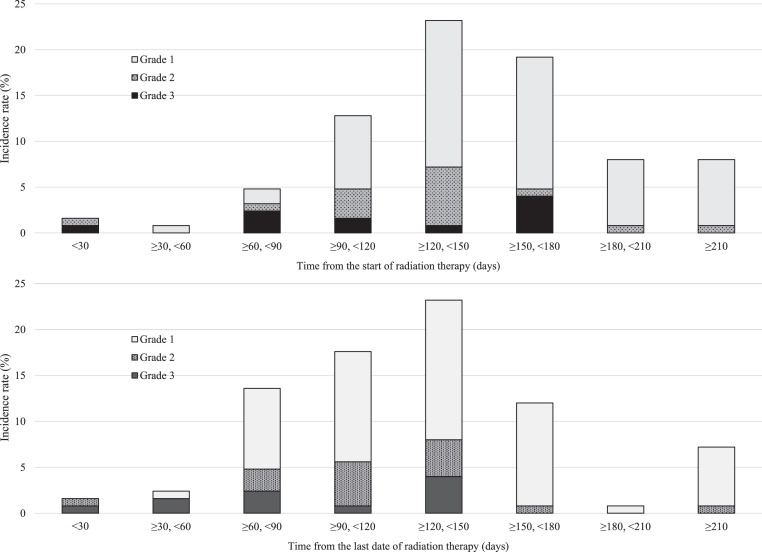
The median follow-up time from the start of CRT was 73.1 months (range, 5.0-177.5 months). Twelve patients developed grade 3 RP, 17 patients developed grade 2 RP, 69 patients developed grade 1 RP, and 27 patients did not exhibit RP based on radiologic findings (ie, grade 0; Table 2). The median time to incidence of RP from initiation of RT was 147 days. Three patients (2.4%) developed RP within 59 days from the start of RT, 6 (4.8%) within 60 to 89 days, 16 (12.8%) within 90 to 119 days, 29 (23.2%) within 120 to 149 days, 24 (19.2%) within 150 to 179 days, and 20 (16.0%) at ≥180 days (Fig. 1).
Table 2.
Grade, initial treatment, and outcomes for RP
| All patients (N = 125) | n | % |
|---|---|---|
| Grade of RP | ||
| 0 | 27 | 21.6 |
| 1 | 69 | 55.2 |
| 2 | 17 | 13.6 |
| 3 | 12 | 9.6 |
| 4 | 0 | 0.0 |
| 5 | 0 | 0.0 |
| Initial treatment of grade ≥2 RP | ||
| Observation without any treatment | 3 | 2.4 |
| Treatment with corticosteroids (initial dose) | 26 | 20.8 |
| Methyl PSL 500-1000 mg/d | 6 | 4.8 |
| PSL 1 mg/kg/d | 4 | 3.2 |
| PSL 0.5 mg/kg/d | 14 | 11.2 |
| PSL 0.3 mg/kg/d | 2 | 1.6 |
| Duration of treatment, day, median (range) | 89 (28-477) | |
| Outcome | ||
| Recovered | 26 | 20.8 |
| Requirement for long-term oxygen therapy | 3 | 2.4 |
PSL, prednisolone; RP, radiation pneumonitis
Fig. 1.
Timing and incidence rate for radiation pneumonitis, showing grade and onset of radiation pneumonitis with timing of onset from A) start of, and B) last date of radiation therapy.
Dose–volume histogram parameters as risk factors for radiation pneumonitis
Correlations between dosimetric factors were assessed using a univariate analysis. Total lung DVH parameters (V5-45 and MLD), except V50 and Dmax, were statistically significantly associated with the incidence of RP grade ≥2 or 3 (as continuous variable; P < .05; Table 3). On univariate analysis, V30 showed the minimal P-value with grade ≥2 RP (P < .0001) and V25 the minimal P-value with grade ≥3 RP (P = .0006). The optimal threshold for V30 to predict RP grade ≥2 was 20%, with a maximum AUC of 0.748, but that of V25 to predict RP grade 3 was 24% (maximum AUC = 0.789). In an analysis of DVHs related to grade ≥2 RP, the maximum AUC for the diagnosis of grade ≥2 RP on ROC analysis was observed using V30 (AUC = 0.748). Therefore, in this study, we selected V30 as a representative parameter for uni- and multivariate analyses for RP of grade ≥2.
Table 3.
Univariate and receiver operating characteristic analyses of total lung dose–volume histogram parameters related to grade ≥2 and 3 RP
| Grade ≥2 RP (n = 29) | Grade <2 RP (n = 96) | Spearman's rank correlation |
Receiver operating characteristic analysis |
Univariate analysis |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Variable, % | Median (interquartile range) | Median (interquartile range) | P-value | rs* | P-value | Area under the curve | Optimal threshold | Crude odds ratio (95% confidence interval) | P-value |
| V5 | 42 (37.5–52) | 35 (25–44.75) | .0054 | 0.787 | < .0001 | 0.671 | 36 | 1.049 (1.008–1.092) | .0146 |
| V10 | 34 (32–44) | 30 (24.25–37) | .0034 | 0.827 | < .0001 | 0.680 | 29 | 1.060 (1.013–1.111) | .0101 |
| V15 | 30 (27.5–37) | 26.5 (22–32) | .0032 | 0.868 | < .0001 | 0.681 | 26 | 1.072 (1.015–1.132) | .0093 |
| V20 | 27 (23.5–30) | 22 (18.25–27) | .0012 | 0.945 | < .0001 | 0.699 | 23 | 1.100 (1.022–1.829) | .0075 |
| V25 | 24 (22–27) | 19 (16.25–23) | .0001 | 0.976 | < .0001 | 0.736 | 21 | 1.140 (1.047–1.241) | .0013 |
| V30 | 20 (20-23.5) | 16 (13.25–20) | < .0001 | ― | ― | 0.748 | 20 | 1.160 (1.058–1.271) | .0006 |
| V35 | 16 (13–17) | 13 (10–15) | .0013 | 0.931 | < .0001 | 0.697 | 16 | 1.144 (1.034–1.265) | .0063 |
| V40 | 13 (10-14.5) | 10 (7–12.75) | .0017 | 0.874 | < .0001 | 0.692 | 13 | 1.169 (1.043–1.311) | .0053 |
| V45 | 6 (4.5–10) | 5 (3–7) | .0103 | 0.672 | < .0001 | 0.657 | 10 | 1.202 (1.050–1.376) | .0063 |
| V50 | 0 (0–0.5) | 0 (0–0) | .3523 | 0.200 | .0258 | 0.538 | 1 | 1.264 (0.534–2.988) | .7348 |
| Dmax | 49.6 (48.7–50.75) | 49.6 (48.5–50.6) | .5608 | 0.325 | .0002 | 0.536 | 49.1 | 1.046 (0.808–1.354) | .6002 |
| MLD, cGy | 1234 (1146–1461) | 1059 (895–1243) | .0008 | 0.939 | < .0001 | 0.705 | 1140 | 1.002 (1.001–1.004) | .0044 |
| Grade ≥3 RP (n = 12) | Grade <3 RP (n = 113) | ||||||||
| V5 (%) | 44 (38–53) | 37.5 (31–45) | .0112 | 0.847 | < .0001 | 0.715 | 36 | 1.060 (1.006–1.117) | .0281 |
| V10 | 37 (32–45) | 32 (25–37.75) | .0098 | 0.882 | < .0001 | 0.719 | 29 | 1.075 (1.011–1.144) | .0187 |
| V15 | 33 (28.5–39) | 27 (22–32.75) | .0089 | 0.914 | < .0001 | 0.722 | 26 | 1.099 (1.020–1.185) | .0101 |
| V20 | 27 (24.5–32.5) | 23 (19–27) | .0039 | 0.986 | < .0001 | 0.745 | 23 | 1.154 (1.038–1.284) | .0045 |
| V25 | 24 (23–29) | 20 (17–23) | .0006 | ― | ― | 0.789 | 22 | 1.205 (1.064–1.364) | .0012 |
| V30 | 21 (20–24) | 17 (14–20) | .0009 | 0.976 | < .0001 | 0.781 | 20 | 1.209 (1.062–1.377) | .0017 |
| V35 | 17 (14.5–18) | 13 (10–16) | .0102 | 0.865 | < .0001 | 0.717 | 16 | 1.169 (1.018–1.341) | .0213 |
| V40 | 13 (11.5–14.5) | 10 (7.25–13) | .0107 | 0.792 | < .0001 | 0.716 | 13 | 1.183 (1.015–1.380) | .0269 |
| V45 | 6 (4–10) | 5 (3–7) | .0842 | 0.589 | < .0001 | 0.646 | 9 | 1.170 (0.985–1.390) | .0767 |
| V50 | 0 (0–0) | 0 (0–1) | .2234 | 0.190 | .0342 | 0.569 | 1 | 1.606 (0.553–4.667) | .4064 |
| MLD | 50.3 (48.35–51.2) | 49.6 (48.6-50.6) | .6360 | 0.283 | .0015 | 0.540 | 50.3 | 1.065 (0.747–1.518) | .7300 |
| MLD, cGy | 1261 (1159–1499) | 1073 (905-1266) | .0055 | 0.961 | < .0001 | 0.736 | 1159 | 1.003 (1.001–1.005) | .0063 |
MLD, maximum lung dose; RP, radiation pneumonitis; Vx, percentage of lung volume receiving >x Gy
Spearman's rank correlation coefficients between V30
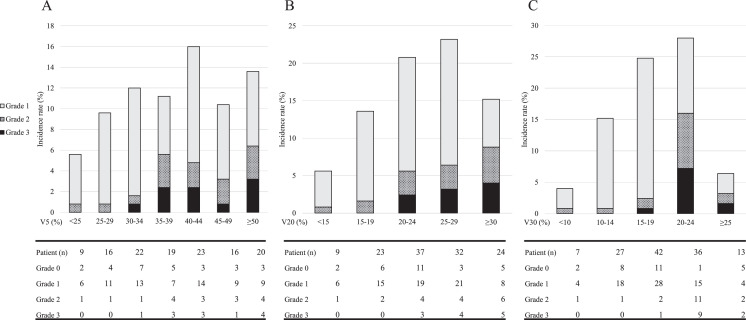
Table 4 summarizes the results of the uni- and multivariate analyses for risk factors related to grade ≥2 RP in enrolled patients. V30 ≥20% (P < .001) was an independent risk factor for RP grade ≥2, with an odds ratio of 13.632 (95% confidence interval, 4.695-39.579; P < .001). The incidence of RP stratified by V5, V20, and V30 is shown in Figure 2. The occurrence rates for RP grade ≥2 in patients with V30 <20% and V30 ≥20% were 6.6% and 49.0%, respectively (Fig. 2). The correlations between total lung DVH parameters and grade ≥2 RP requiring steroids were also evaluated. The results were the same as those obtained in the analysis of grade 2 RP, and are presented in the Supplementary Table.
Table 4.
Uni- and multivariate analyses of risk factors for grade ≥2 radiation pneumonitis
| Variables (N = 125) | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| Odds ratio (95% confidence interval) | P-value | Odds ratio (95% confidence interval) | P-value | |
| Age (<65/≥65 y) | 0.414 (0.167–1.026) | .0490 | 0.456 (0.164-1.270) | .133 |
| Sex (male/female) | 1.167 (0.446–3.056) | .751 | ― | ― |
| Eastern Cooperative Oncology Group performance status score (0/1) | 1.086 (0.471–2.502) | .846 | ― | ― |
| Smoking status (past/current) | 0.667 (0.266–1.670) | .387 | ― | ― |
| Diabetes mellitus (yes/no) | 3.076 (1.031–9.174) | .0439 | 1.559 (0.423-5.742) | .505 |
| Percentage of lung volume receiving >30 Gy (≥20%/<20%) | 13.632 (4.695–39.579) | < .001 | 12.352 (4.127-36.973) | < .001 |
Fig. 2.
Incidence rate of radiation pneumonitis, stratified by total lung dose–volume histogram findings, shown by percentage of long volume receiving A) >5 Gy, B) >20 Gy, and C) >30 Gy.
Treatment and outcomes for radiation pneumonitis
Of the 29 patients with RP grade ≥2, 26 received corticosteroids. The initial dose was as follows: Methylprednisolone at 500 to 1000 mg/d (n = 6), prednisolone (PSL) at 0.3 mg/kg/d (n = 2), PSL at 0.5 mg/kg/d (n = 14), and PSL at 1 mg/kg/d (n = 4). The median duration of treatment was 89 days (range: 28-477 days). The 3 patients who did not receive any treatment were considered to have grade 2 pneumonitis because they showed abnormal lung shadows in and around the radiation field and experienced increased shortness of breath and coughing. Oxygen desaturation was also observed in 2 of the 3 patients. From these patients, 2 recovered with observation alone, and 1 showed radiation fibrosis on lung CT. Twenty-six patients recovered, 1 patient required long-term oxygen therapy, and no treatment-related deaths were observed.
Discussion
To the best of our knowledge, this is the largest retrospective study of patients with LS-SCLC treated with CRT using AHF-RT to evaluate DVH parameters for risk factors and investigate the onset, treatment, and outcomes for RP. V20 was previously reported as a risk factor for RP in patients with LA-NSCLC treated with CRT.9,10,13,14,16 In patients with LS-SCLC receiving CRT using AHF-RT, V20 was also verified as a predictor of RP in several retrospective studies.5,23,24 Only one retrospective study that enrolled a small number of patients with LS-SCLC evaluated various DVH parameters.5 In the present study, V30 was considered a higher-level risk factor for RP among the evaluated DVH parameters. Therapeutic efficacy is expected to be maximized with the administration of radiation twice daily, but reducing the dose per fraction and maintaining a larger tolerance dose to normal tissues.24,25 Therefore, V30, which represents a higher dose range than V20, is thought to be a risk factor for RP in patients with LA-NSCLC treated with CRT. Moreover, V30 was verified as a risk factor for RP in patients with LS-SCLC treated with AHF-RT. The effect of each DVH parameter on RP may differ between AHF-RT and conventional irradiation. In this study, we found that V30 ≥20% could consistently distinguish symptomatic RP overall. According to our findings, V25 ≥22% may potentially distinguish RP grade ≥3.
Furthermore, we found that the incidence of RP gradually increased 60 days after the start of radiation, with the highest rate evidenced at 120 to 150 days. The median time to the onset of RP was 147 days after the initiation of RT. Previous studies on CRT-induced RP in patients with LA-NSCLC showed that the median time to the onset of RP from the time of initiation of RT was 92 to 123 days,11,19,25 and that the median time to the onset of RP in patients with LS-SCLC treated with concurrent CRT using AHF-RT was 5 months from the initiation of RT.24 Although the median time from the last date of RT to the onset of RP was 123 days in the present study, previous studies evaluating RP in patients with LA-NSCLC showed a median onset time of 2.0 to 3.4 months.10,26 Therefore, the onset of RP induced by AHF-RT in patients with LS-SCLC may be later than that induced by conventional irradiation in patients with LA-NSCLC. There may be a difference in the incidence of RP between patients with LS-SCLC treated with AHF-RT and patients with LA-NSCLC treated with conventional irradiation, according to tumor location or chemotherapy regimen.
Recent randomized phase 2 or 3 trials of patients with LS-SCLC treated with CRT have reported the following rates in the AHF-RT group: 5% to 20% for grade 1 to 2 RP, and 0% to 1% for grade 3 RP.3,27,28 A previous Chinese randomized phase 2 trial found a relatively high incidence of RP with the following reported rates: 14.1% for grade 2 RP, 2.2% for grade 3 RP, and 1.1% for grade 5 RP.29 Possible reasons for the higher frequency of RP detected in this study may be attributed to different ethnic backgrounds, as well as the effects of elective nodal irradiation. However, of note, AHF-RT for LS-SCLC can be performed safely despite the high frequency of RP observed in this study.
Although SCLC is one of the most aggressive tumors, LS-SCLC is curable with CRT.1,2 Among patients with LS-SCLC treated with CRT, the reported rates of locoregional recurrences and distant metastases were 15% to 26% and 44.6-54.6%, respectively.23,30,31 Inhibiting distant metastasis with additional systemic therapy on the administration CRT could be important to improve the prognosis of patients with LS-SCLC. An ongoing randomized clinical study is evaluating the efficacy of the addition of immune checkpoint inhibitors to CRT.32 Our study results show that the addition of immune checkpoint inhibitors to CRT for patients with LS-SCLC may be tolerable.
To our knowledge, this is the first study to report on the treatment of and outcomes for RP in patients with LS-SCLC treated with concurrent CRT using AHF-RT. The severity of RP in our enrolled patients was relatively mild, and no deaths due to RP were recorded. Patients with RP responded well to steroid therapy and improved without sequelae, with the exception of 1 patient who required oxygen therapy. Remarkably, RP was not exacerbated during treatment.
We acknowledge some limitations of this study. First, the single-center retrospective design of this study may confer some level of bias and likewise affect generalizability. Second, RP treatment was based on each physician's discretion. Additional prospective studies are necessary to evaluate the optimal dose and/or treatment duration for corticosteroid treatment to standardize the treatment of RP.
Conclusions
RP was observed in 78.3% of patients with LS-SCLC treated with concurrent CRT using AHF-RT, and RP of grade ≥2 was observed in 21.6% of our enrolled patients. The onset of RP induced by concurrent CRT using AHF-RT in patients with LS-SCLC might occur later than that induced by conventional irradiation in patients with LA-NSCLC. Among the evaluated DVH parameters, V30 was the most strongly associated with RP in patients with LS-SCLC treated with AHF-RT. There were no deaths due to RP within the present study and a good response to steroid therapy was observed, indicating that RP induced by CRT using AHF-RT is manageable in patients with LS-SCLC.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: Dr Kenmotsu reports grants and personal fees from AstraZeneca K.K., Chugai Pharmaceutical Co., Daiichi-Sankyo Co., Ltd., and Novartis Pharma K.K.; grants from Loxo Oncology; and personal fees from Ono Pharmaceutical Co, Ltd., Boehringer Ingelheim, Eli Lilly K.K., Kyowa Hakko Kirin Co., Ltd., Bristol-Myers Squibb, Pfizer Inc, and Taiho Pharmaceutical outside of the submitted work. Dr Omori reports grants and personal fees from Daiichi-Sankyo Co., Ltd. and personal fees from Chugai Pharmaceutical Co., AstraZeneca K.K., Ono Pharmaceutical Co., Ltd., Novartis Pharma K.K., Taiho Pharmaceutical, Eli Lilly Japan K.K., Daiichi Sankyo Co., Ltd., and Amgen K.K. outside of the submitted work. Dr Mamesaya reports grants and personal fees from Boehringer Ingelheim and personal fees from Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical, MSD K.K., AstraZeneca K.K., and Ono Pharmaceutical Co., Ltd. outside of the submitted work. Mr Kobayashi reports personal fees from Eli Lilly K.K., Novartis Pharma K.K., Taiho Pharmaceutical., AstraZeneca K.K., Chugai Pharmaceutical Co., LTD., and Ono Pharmaceutical Co., LTD. outside of the submitted work. Dr Ko reports grants and personal fees from Boehringer Ingelheim; grants from MSD K.K.; and personal fees from Taiho Pharmaceutical, Chugai Pharmaceutical Co., Ltd., Eli Lilly K.K., Boehringer Ingelheim, Pfizer Inc, and AstraZeneca K.K. outside of the submitted work. Dr Wakuda reports grants and personal fees from Chugai Pharmaceutical Co., Ltd. and AstraZeneca K.K.; grants from Novartis Pharma K.K., Abbvie, and Amgen; and personal fees from Taiho Pharmaceutical, Boehringer Ingelheim, Eli Lilly K.K., Ono Pharmaceutical Co., Ltd., and MSD outside of the submitted work. Dr Ono reports personal fees from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., and Ono Pharmaceutical Co., Ltd. outside of the submitted work. Dr Naito reports grants from Otsuka Pharmaceutical K.K. and personal fees from Ono Pharmaceutical Co., Ltd. And Helsinn Health care SA outside of the submitted work. Dr Murakami reports grants and personal fees from AstraZeneca K.K., Takeda Pharmaceutical Co., Ltd., and Daiichi-Sankyo Co., grants from Abbvie; grants from IQvia; and personal fees from Chugai Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb Japan, MSD, Pfizer Inc, Novartis Pharma K.K., Eli Lilly Japan K.K., and Taiho Pharmaceutical of outside the submitted work. Dr Harada reports personal fees from AstraZeneca K.K., Brainlab, Pfizer Japan Inc, MSD, and Eli Lilly Japan K.K. outside of the submitted work. Dr Kaneko reports grants and personal fees from Boehringer Ingelheim; grants from Eli Lilly Japan K.K. and Taiho Pharmaceutical; and personal fees from GlaxoSmithKline, AstraZeneca K.K., Novartis Pharma K.K., Nippon Kasei Chemical Co., Ltd., and Takeda Pharmaceutical Co. outside of the submitted work. Dr Takahashi reports grants and personal fees from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Ono Pharmaceutical Co., Ltd., MSD, Pfizer Japan Inc, and Boehringer Ingelheim Japan; grants from Merck Biopharma Co., Ltd. and Amgen Inc; and personal fees from Roche Diagnostics K.K., Takeda Pharmaceutical Co., Ltd., and Yakult Honsha Co. Ltd. outside of the submitted work. All other authors have no disclosures to declare.
Data sharing statement: Research data are available from the corresponding author upon reasonable request.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.adro.2022.101129.
Appendix. Supplementary materials
References
- 1.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 2.Kubota K, Hida T, Ishikura S, et al. Etoposide and cisplatin versus irinotecan and cisplatin in patients with limited-stage small-cell lung cancer treated with etoposide and cisplatin plus concurrent accelerated hyperfractionated thoracic radiotherapy (JCOG0202): A randomised phase 3 study. Lancet Oncol. 2014;15:106–113. doi: 10.1016/S1470-2045(13)70511-4. [DOI] [PubMed] [Google Scholar]
- 3.Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): An open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18:1116–1125. doi: 10.1016/S1470-2045(17)30318-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turrisi AT, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 5.Tsujino K, Hirota S, Kotani Y, et al. Radiation pneumonitis following concurrent accelerated hyperfractionated radiotherapy and chemotherapy for limited-stage small-cell lung cancer: Dose-volume histogram analysis and comparison with conventional chemoradiation. Int J Radiat Oncol Biol Phys. 2006;64:1100–1105. doi: 10.1016/j.ijrobp.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Ha IB, Jeong BK, Jeong H, et al. Effect of early chemoradiotherapy in patients with limited stage small cell lung cancer. Radiat Oncol J. 2013;31:185. doi: 10.3857/roj.2013.31.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asakura H, Hashimoto T, Zenda S, et al. Analysis of dose–volume histogram parameters for radiation pneumonitis after definitive concurrent chemoradiotherapy for esophageal cancer. Radiother Oncol. 2010;95:240–244. doi: 10.1016/j.radonc.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Wang D, Sun J, Zhu J, Li X, Zhen Y, Sui S. Functional dosimetric metrics for predicting radiation-induced lung injury in non-small cell lung cancer patients treated with chemoradiotherapy. Radiat Oncol. 2012;7:69. doi: 10.1186/1748-717X-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsujino K, Hirota S, Endo M, et al. Predictive value of dose-volume histogram parameters for predicting radiation pneumonitis after concurrent chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2003;55:110–115. doi: 10.1016/s0360-3016(02)03807-5. [DOI] [PubMed] [Google Scholar]
- 10.Shintani T, Kishi N, Matsuo Y, et al. Incidence and risk factors of symptomatic radiation pneumonitis in non–small-cell lung cancer patients treated with concurrent chemoradiotherapy and consolidation durvalumab. Clin Lung Cancer. 2021;22:401–410. doi: 10.1016/j.cllc.2021.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Saito G, Oya Y, Taniguchi Y, et al. Real-world survey of pneumonitis and its impact on durvalumab consolidation therapy in patients with non-small cell lung cancer who received chemoradiotherapy after durvalumab approval (HOPE-005/CRIMSON) Lung Cancer. 2021;161:86–93. doi: 10.1016/j.lungcan.2021.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Ramella S, Trodella L, Mineo TC, et al. Adding ipsilateral V20 and V30 to conventional dosimetric constraints predicts radiation pneumonitis in stage IIIA-B NSCLC treated with combined-modality therapy. Int J Radiat Oncol Biol Phys. 2010;76:110–115. doi: 10.1016/j.ijrobp.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 13.Palma DA, Senan S, Tsujino K, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: An international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85:444–450. doi: 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC) Int J Radiat Oncol Biol Phys. 1999;45:323–329. doi: 10.1016/s0360-3016(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 15.Fay M, Tan A, Fisher R, MacManus M, Wirth A, Ball D. Dose–volume histogram analysis as predictor of radiation pneumonitis in primary lung cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1355–1363. doi: 10.1016/j.ijrobp.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Hope AJ, Lindsay PE, El Naqa I, et al. Modeling radiation pneumonitis risk with clinical, dosimetric, and spatial parameters. Int J Radiat Oncol Biol Phys. 2006;65:112–124. doi: 10.1016/j.ijrobp.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 17.Tsujino K, Hashimoto T, Shimada T, et al. Combined analysis of V20, VS5, pulmonary fibrosis score on baseline computed tomography, and patient age improves prediction of severe radiation pneumonitis after concurrent chemoradiotherapy for locally advanced non–small-cell lung cancer. J Thor Oncol. 2014;9:983–990. doi: 10.1097/JTO.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 18.Hernando ML, Marks LB, Bentel GC, et al. Radiation-induced pulmonary toxicity: A dose–volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:650–659. doi: 10.1016/s0360-3016(01)01685-6. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Z, Song X, Wu A, et al. Pulmonary emphysema is a risk factor for radiation pneumonitis in NSCLC patients with squamous cell carcinoma after thoracic radiation therapy. Sci Rep. 2017;7:2748. doi: 10.1038/s41598-017-02739-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ergen SA, Dincbas FO, Yücel B, et al. Risk factors of radiation pneumonitis in patients with NSCLC treated with concomitant chemoradiotherapy—Are we underestimating diabetes?—Turkish Oncology Group (TOG)/Lung Cancer Study Group. Clin Respir J. 2020;14:871–879. doi: 10.1111/crj.13220. [DOI] [PubMed] [Google Scholar]
- 21.Kong M, Lim YJ, Kim Y, et al. Diabetes mellitus is a predictive factor for radiation pneumonitis after thoracic radiotherapy in patients with lung cancer. Cancer Manag Res. 2019;11:7103–7110. doi: 10.2147/CMAR.S210095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Cancer Institute, Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events. Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Accessed October 4, 2022.
- 23.Sas-Korczyńska B, Łuczyńska E, Kamzol W, Sokołowski A. Analysis of risk factors for pulmonary complications in patients with limited-stage small cell lung cancer: A single-centre retrospective study. Strahlenther Onkol. 2017;193:141–149. doi: 10.1007/s00066-016-1069-6. [DOI] [PubMed] [Google Scholar]
- 24.Giuliani ME, Lindsay PE, Kwan JYY, et al. Correlation of dosimetric and clinical factors with the development of esophagitis and radiation pneumonitis in patients with limited-stage small-cell lung carcinoma. Clin Lung Cancer. 2015;16:216–220. doi: 10.1016/j.cllc.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Horinouchi H, Atagi S, Oizumi S, et al. Real-world outcomes of chemoradiotherapy for unresectable stage III non-small cell lung cancer: The SOLUTION study. Cancer Med. 2020;9:6597–6608. doi: 10.1002/cam4.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaverdian N, Thor M, Shepherd AF, et al. Radiation pneumonitis in lung cancer patients treated with chemoradiation plus durvalumab. Cancer Med. 2020;9:4622–4631. doi: 10.1002/cam4.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grønberg BH, Killingberg KT, Fløtten Ø, et al. High-dose versus standard-dose twice-daily thoracic radiotherapy for patients with limited stage small-cell lung cancer: An open-label, randomised, phase 2 trial. Lancet Oncol. 2021;22:321–331. doi: 10.1016/S1470-2045(20)30742-7. [DOI] [PubMed] [Google Scholar]
- 28.Grønberg BH, Halvorsen TO, Fløtten Ø, et al. Randomized phase II trial comparing twice daily hyperfractionated with once daily hypofractionated thoracic radiotherapy in limited disease small cell lung cancer. Acta Oncol. 2016;55:591–597. doi: 10.3109/0284186X.2015.1092584. [DOI] [PubMed] [Google Scholar]
- 29.Qiu B, Li Q, Liu J, et al. Moderately hypofractionated once-daily compared with twice-daily thoracic radiation therapy concurrently with etoposide and cisplatin in limited-stage small cell lung cancer: A multicenter, phase II, randomized trial. Int J Radiat Oncol Biol Phys. 2021;111:424–435. doi: 10.1016/j.ijrobp.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Reymen B, Van Loon J, van Baardwijk A, et al. Total gross tumor volume is an independent prognostic factor in patients treated with selective nodal irradiation for stage I to III small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;85:1319–1324. doi: 10.1016/j.ijrobp.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Maranzano E, Crinò L, Piro F, et al. Long-term results of induction chemotherapy followed by concurrent chemotherapy and thoracic irradiation in limited small cell lung cancer. Lung Cancer. 2002;37:79–85. doi: 10.1016/s0169-5002(02)00028-4. [DOI] [PubMed] [Google Scholar]
- 32.Senan S, Okamoto I, Lee GW, et al. Design and rationale for a Phase III, randomized, placebo-controlled trial of durvalumab with or without tremelimumab after concurrent chemoradiotherapy for patients with limited-stage small-cell lung cancer: The Adriatic study. Clin Lung Cancer. 2020;21:e84–e88. doi: 10.1016/j.cllc.2019.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.