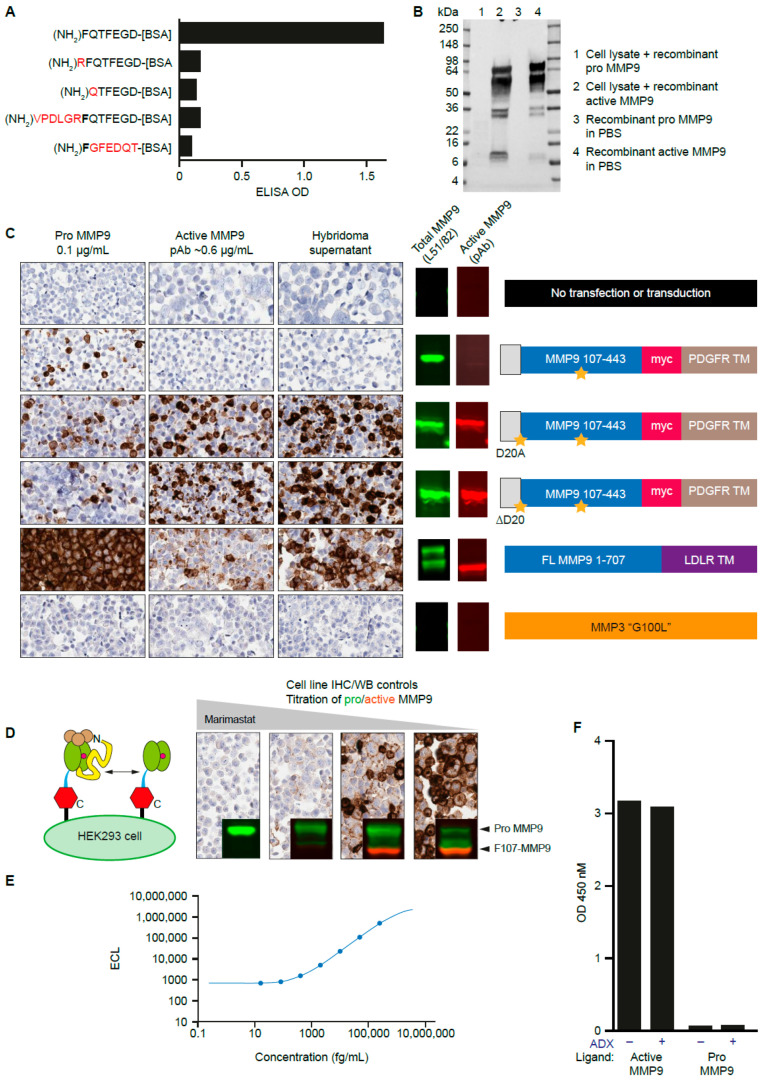
Figure 2.
Discovery and characterization of antibodies directed at the active MMP9 neoepitope. (A) Hybridoma supernatants specific for the N-terminus of MMP9 after cleavage between Arg106 and Phe107 were identified by direct ELISA toward the corresponding on-target and off-target peptides. (B) Pro-MMP9 and active-MMP9 protein standards in buffer or cell lysate were probed with candidate hybridoma supernatants by Western blot. The multiple bands represent the multiple degradation products of MMP9. (C) Antibodies that reacted with FFPE cell pellets engineered to contain the active MMP9 neoepitope were identified. Orange stars indicate point mutations, either at the signal peptide junction with F107 (D20A or D20 deletion engineered to generate the F107 neoepitope; see Appendix A) or a mutation in the catalytic domain rendering MMP9 inactive. PDGFR TM and LDLR TM refer to transmembrane domains of PDGFR and LDLR, respectively. MMP3 G100L is an active form of MMP3, a negative control. Molecular weights of bands shown correspond to Figure 1. (D) Specificity of the candidate IHC antibodies were determined using cell pellets in which the pro-MMP9:active MMP9 ratio was titrated using marimastat (0.025–2.5 μM). Inset Western blot images fall between the 98 and 62 kDa molecular weight markers. (E) A sandwich ELISA using anti–active MMP9 antibodies paired with total MMP9 antibody detected active MMP9 in diluent with a sensitivity <1 pg/mL. (F) ADX does not interfere with the sandwich ELISA for active MMP9. ADX, andecaliximab; BSA, bovine serum albumin; ECL, electrochemiluminescence; ELISA, enzyme-linked immunosorbent assay; FFPE, formalin-fixed/paraffin-embedded; IHC, immunohistochemistry; LDLR, low-density lipoprotein receptor; MMP, matrix metalloproteinase; OD, optical density; PDGFR, platelet-derived growth factor receptor; PBS, phosphate-buffered saline; TM, transmembrane; WB, Western blot.