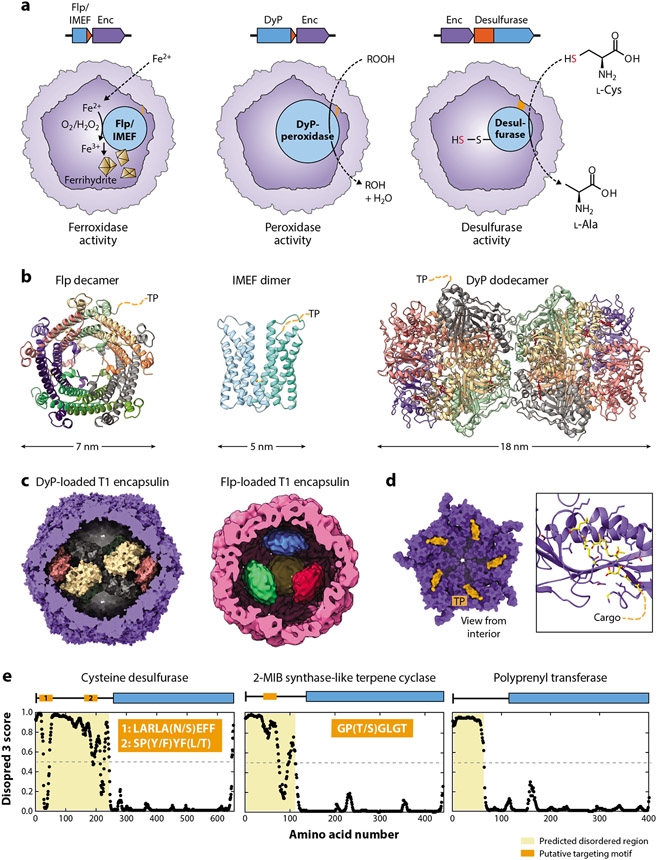
Figure 5.
Cargo proteins and encapsulin biochemistry. (a) Overview of the three so-far partially characterized biochemical functions of encapsulins, namely, ferroxidase, peroxidase, and desulfurase activity. TPs/domains are highlighted in orange. (b) Structures of cargo proteins highlighting their oligomerization state. For clarity, only one TP per oligomer is shown. DyP-bound heme groups are shown in red. (c) DyP (Mycobacterium smegmatis) and Flp (Haliangium ochraceum) cargo proteins shown in their native assembly state inside cargo-loaded T1 encapsulins. (d) Family 1 cargo-loading mechanism mediated by TP binding to the interior of the encapsulin shell. (Left) A pentameric facet and (right) a zoomed-in view on a single binding site are shown (Thermotoga maritima; 3DKT). TPs are shown in orange/yellow. Residues within 5 Å of the TP are shown as sticks. (e) Proposed cargo-loading mechanism for Family 2 encapsulins. Shown are disorder plots generated by Disopred 3 highlighting the large, disordered N-terminal regions/domains in many putative Family 2 cargo proteins. Three examples (desulfurase, terpene cyclase, and polyprenyl transferase) are shown with proposed targeting motifs indicated in orange. Abbreviations: DyP, dye-decolorizing peroxidase; Enc, encapsulin; Flp, ferritin-like protein; IMEF, iron-mineralizing encapsulin-associated firmicute; MIB, methylisoborneol; TP, targeting peptide.