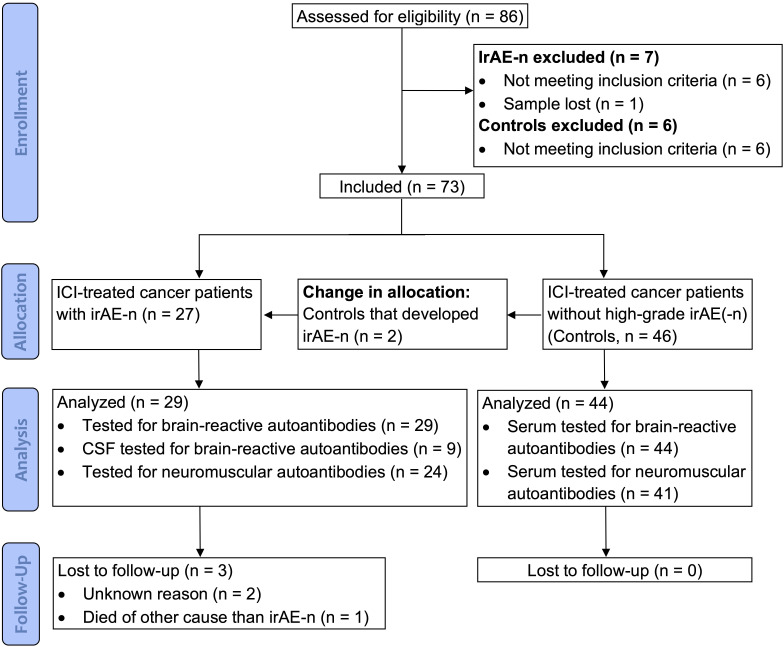
Figure 1.
CONSORT diagram. Between September 2017 and January 2022, a total of 86 cancer patients were enrolled for the study. After application of inclusion and exclusion criteria, 73 patients were included. As two patients that were originally allocated to the control cohort developed irAE-n, a total of 29 irAE-n patients and 44 cancer control patients were analyzed. CSF, cerebrospinal fluid; ICI, immune checkpoint inhibitor; irAEs, immune related adverse events; irAE-n, neurological immune related adverse event.