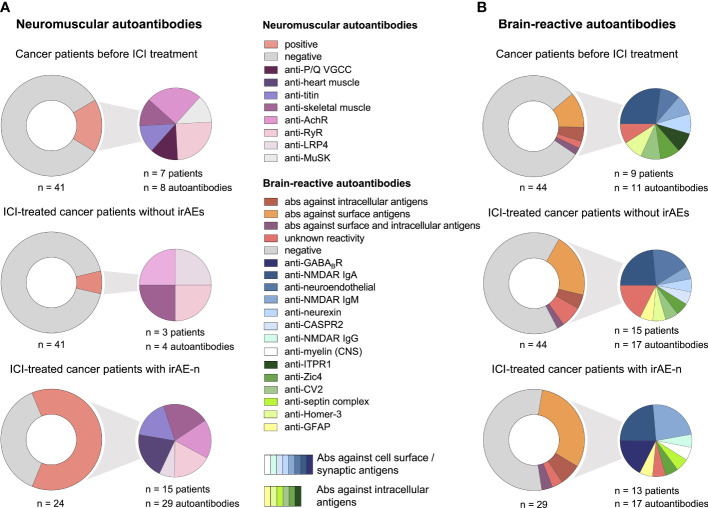
Figure 2.
Serum autoantibody profiles in cancer patients with irAE-n and controls. (A) Frequency and specification of neuromuscular autoantibodies of 41 cancer patients before (upper left) and six weeks after (middle left) ICI treatment initiation who did not develop high-grade (= CTCAE ≥ 3) irAEs and 24 ICI-treated cancer patients with irAE-n (lower left). (B) Frequency and specification of brain-reactive autoantibodies of 44 cancer patients before (upper right) and six weeks after (middle right) ICI treatment initiation who did not develop high-grade (CTCAE ≥ 3) irAEs and 29 ICI-treated cancer patients with irAE-n (lower right). Ab, autoantibody; AchR, acetylcholine receptor; CASPR2, contactin-associated protein-like 2; GABABR, gamma-aminobutyric-acid B receptor; GFAP, glial fibrillary acidic protein; Homer-3, Homer protein homolog 3; ICI, immune checkpoint inhibitor; irAEs, immune related adverse events; irAE-n, neurological immune related adverse event; ITPR1, inositol 1,4,5-trisphosphate receptor 1; LRP4, low-density lipoprotein receptor-related protein 4; MuSK, muscle-specific tyrosine kinase; NMDAR, N-methyl-D-aspartate receptor; P/Q VGCC, P/Q-type voltage gated calcium channel; RyR, ryanodine receptor; Zic4, zinc finger 4.