Abstract
Background:
Generalized anxiety disorder (GAD) and major depressive disorder (MDD) reliably precede and predict one another. However, there is insufficient data on mediators through which the longitudinal GAD-MDD association unfold. Based on insomnia theories, such as the hyperarousal model of sleep, we tested the degree to which poor global sleep quality functioned as a mediator of the prospective bidirectional anxiety-depression relationship.
Method:
Participants were 3,294 community-dwelling adults who partook in three measurement waves nine years apart. The Composite International Diagnostic Interview-Short Form assessed GAD and MDD in-person at baseline (Time 1 [T1]), Time 2 (T2; nine years after T1), and 18 years later (T3). T2 global sleep quality was measured using the multiple-domain Pittsburgh Sleep Quality Index self-report at T2. We used longitudinal structural equation modeling mediation analyses.
Results:
Analyses showed that higher T1 MDD and GAD severity individually predicted lower T2 global sleep quality (Cohen’s d = −0.561 to −0.480) and less T2 global sleep quality, thereby forecasted both higher T3 MDD and GAD (d = −0.275 to −0.190). Poorer T2 global sleep quality significantly mediated the T1 GAD–T3 MDD relation, explaining 41% of the association. Worse global sleep quality at T2 also significantly mediated the T1 MDD–T3 GAD association, mediating 11% of the T1 MDD–T3 GAD pathway. The results remained similar after controlling for multiple sociodemographic and clinical variables.
Conclusions:
Findings offer evidence for transdiagnostic theories of sleep and insomnia. Theoretical and clinical implications, such as prioritizing sleep improvement in cognitive-behavioral therapies, are also discussed.
Keywords: Sleep quality, Depression, Anxiety, Longitudinal, Mediator, Comorbidity
Why Sleep is Key: Poor Sleep Quality is a Mediator for the Bidirectional Relationship between Major Depressive Disorder and Generalized Anxiety Disorder Across 18 Years
Approximately one-fifth of the population suffers from anxiety or depressive disorders in a given year (cf. recent meta-analysis on global prevalence rates of mental disorders; Steel et al., 2014). Further, 49 to 81% of those suffering from a depressive disorder also meet diagnostic criteria for an anxiety disorder in their lifetime, and about 47 to 88% of anxiety-disordered individuals are affected by depressive disorders (Regier et al., 1998). When compared with either diagnosis alone, co-morbid diagnoses of major depressive disorder (MDD) and generalized anxiety disorder (GAD) coincided with greater symptom severity, chronicity, and impairment (Kessler et al., 2008). Simultaneously, MDD and GAD were associated with heightened interpersonal distress (Uhmann et al., 2010), lower annual income (Kessler & Heeringa, 2008), and a plethora of physical ailments related to immune, cardiorespiratory, and neurocognitive disorders (Butnoriene et al., 2015; Zainal & Newman, 2018, 2021a, 2021b, 2021, 2022). Due to the consequences of its comorbidity, a better understanding of the MDD-GAD relation is essential.
On top of the wealth of research demonstrating concurrent MDD-GAD relations, abundant evidence suggests that the association holds across multiple time-lags. Prospective comorbidity theories have proposed that anxiety can evoke hopelessness and helplessness, prompting future depression (Swendsen, 1997). Similarly, anxiety theorists have posited that higher levels of anxiety result in greater levels of inhibition and prolonged behavioral inactivity, which over time evokes depression (Rosellini & Brown, 2011). Supporting those theories, a recent meta-analysis by (Jacobson & Newman, 2017) observed equally large effect sizes for anxiety disorders predicting future disorders of depression and vice versa. However, the mechanisms underlying the longitudinal MDD-GAD comorbidity remain understudied.
One potential mechanism could be sleep. The transdiagnostic cognitive theory of sleep posits that insomnia is a candidate mediator in explaining the longitudinal relation between GAD and MDD across extended timeframes (Harvey, 2008). The theory asserts that over protracted timescales, insomnia can mediate the GAD-MDD association as it decreases quality of life due to economic and physical health consequences, such as work absenteeism and over-reliance on medical services. Moreover, sleep deprivation may reduce one’s ability to manage emotions, and acute stress triggers, contributing to the onset and maintenance of psychological disorders over time. Chronic sleep issues could also contribute to emotional difficulties via executive dysfunction (e.g., impaired working memory) and problems focusing, multitasking, and accomplishing tasks (Grima et al., 2019). However, depression and anxiety disorders can also aggravate sleep patterns in the long term (Gregory et al., 2005; Morphy et al., 2007). Collectively, theory and evidence thus far suggest a reciprocal prospective connection between insomnia and anxiety or depressive disorders and symptoms (e.g., up to as long as 50 years) (Jansson-Fröjmark & Lindblom, 2008; Kim et al., 2009; Sivertsen et al., 2012).
Further, the hyperarousal model of insomnia (Riemann et al., 2010) postulates that insomnia is a psychobiological disorder involving psychological disturbances and biological imbalances across extended time-points. Psychological disturbances may manifest as maladaptive sleep behaviors, creating associations between sleep and negative mood, excessive worry, rumination, and dysfunctional beliefs and attitudes toward sleep. Such psychological factors tend to interact with physical disharmony of the neuroendocrine and immunological systems (e.g., excessive cortisol, insulin, and pro-inflammatory cytokines such as interleukin-6) (Cho et al., 2015; O’Connor et al., 2014; Stahl et al., 2020; Zainal & Newman, 2021a; in press). It is also plausible that elevated sympathetic tone, reduced heart rate variability (indicative of cognitive rigidity in response to acute stress), and dysregulated hypothalamic-pituitary axis (HPA) could mediate the links between chronic sleep troubles, MDD, and GAD over time (Chalmers et al., 2022; Dodds et al., 2017). On the whole, the model proposes that wear-and-tear of biological systems (e.g., HPA) from poor sleep habits can account for the bidirectional association between poor sleep quality and depression and anxiety symptoms across long periods.
Five longitudinal studies thus far have examined the proposition that poorer sleep quality precedes and predicts anxiety or depressive symptoms. For example, sleep difficulties contributed to the onset, relapse, and maintenance of anxiety and depression disorders following nine years in young adult women (Jackson et al., 2014). Moreover, at one-year follow-up, poor sleep quality at baseline predicted a higher risk of anxiety symptoms among Chinese male college students (Zou et al., 2020). Likewise, more inferior sleep quality forecasted anxiety and depression across time in pregnant women (Yu et al., 2017). Another study found no significant association between sleep problems and higher depressive symptoms 14 months later in depressed cancer survivors (Hsiao et al., 2013). Relatedly, among Swiss adults, there was a prospective link between insomnia and future depressive episodes across 20 years (Buysse et al., 2008). Based on these findings, it is plausible that lower global sleep quality could precede and predict MDD and GAD.
Additionally, four studies thus far investigated if anxiety and depressive disorders predicted various sleep problems at a later time. For instance, higher anxiety severity predicted poorer sleep quality and shorter sleep duration, after 1.5 years, in adolescents who survived the 2008 Wenchuan earthquake (Geng et al., 2018). Similarly, another study found a prospective relationship between depressive symptoms and sleep disturbance (i.e., shorter vs. longer sleep duration) over four years (Sun et al., 2018); however, this study limited its sleep disturbance measure to sleep duration. Further, among twins, although sleep problems (i.e., difficulties falling or staying asleep, excessive sleep, or issues with experiencing restful sleep) predicted depressive symptoms after two years, the reverse relationship did not occur (Gregory et al., 2005). In addition, state anxiety and depressive symptoms at baseline predicted the incidence of insomnia six months later in patients with Parkinson’s Disease (PD) (Rutten et al., 2017). Collectively, these findings support the idea that anxiety and depressive disorders can predict future sleep problems across long durations.
Building on existing research, the current study aimed to investigate if subjective sleep quality problems assessed during mid-point mediated the 18-year longitudinal relations between MDD and GAD severity. The present study adds to the literature in several ways. First, the current literature consists of mostly cross-sectional studies, which preclude causal inferences due to the absence of temporal precedence. Our 18-year longitudinal study contributes to a potential cause-effect understanding of the bi-directional relations between sleep problems and MDD or GAD. Second, our study examined community-dwelling adults using a thorough psychiatric diagnostic interview. Previous studies investigating the bidirectional MDD–GAD relationship included scales utilizing limited clinical diagnostic criteria (Jansson-Fröjmark & Lindblom, 2008). Third, we used a measure of global sleep quality that contained different facets of the sleep experience. Fourth, this is the first study to examine if overall sleep quality was a mediator (or mechanism) of the 18-year relation between MDD and GAD severity.
Based on theory and research, we predicted that higher MDD and GAD symptom severity would forecast reduced future global sleep quality (indexed by subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, presence of use of sleep medications, daytime dysfunction) 9 years later. Moreover, we hypothesized that lower global sleep quality would predict greater MDD and GAD symptom severity following nine years. In addition, we hypothesized that global sleep quality measured at mid-point would mediate the 18-year relationship between MDD and GAD symptom severity.
1. Method
1.1. Participants
Participants comprised 3294 community-dwelling adults who consented to take part in the Midlife Development in the United States (MIDUS) project at Time 1 (T1) (1995–1996), T2 (2004–2005), and T3 (2013–2014) (Brim et al., 2019; Ryff et al., 2017, 2019). Participants were aged 45.62 years on average (SD = 11.41, range = 20–74 years) and 92.93% identified as White compared to African American, Hispanic, Asian, Pacific Islander, or others. Concerning gender, 54.61% were women, 44.84% were men, and the remaining 0.55% declined to disclose their gender identity. Since this study is a secondary analysis of a publicly available dataset, it was exempt from institutional review board approval. Table 1 presents the descriptive statistics and correlation matrix of the study variables.
Table 1.
Correlation Matrix of Study Variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. Age | – | ||||||
| 2. Gender | −0.002 | – | |||||
| 3. T1 MDD | −0.092*** | .075*** | – | ||||
| 4. T1 GAD | −0.044* | .075*** | .288*** | – | |||
| 5. T3 MDD | −0.088*** | .083*** | .279*** | .182*** | – | ||
| 6. T3 GAD | −0.086*** | .066*** | .159*** | .354*** | .340*** | – | |
| 7. T2 PSQI | −0.041 | .144*** | .224*** | .179*** | .291*** | .181*** | – |
| M or % | 45.62 | 54.61 | 0.69 | 0.14 | 0.6 | 0.13 | 6.09 |
| SD | 11.41 | – | 1.82 | 0.86 | 1.71 | 0.92 | 3.58 |
| Min | 20.00 | – | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Max | 74.00 | – | 7.00 | 10.00 | 7.00 | 10.00 | 19.00 |
| Skewness | 0.24 | – | 2.44 | 7.47 | 2.69 | 7.89 | 0.93 |
| Kurtosis | −0.70 | – | 4.35 | 60.84 | 5.74 | 65.66 | 0.57 |
Note.
p < .05;
p < .001.
GAD = generalized anxiety disorder; PSQI = Pittsburgh Sleep Quality Index; MDD = major depressive disorder; M = mean; Max = maximum; Min = minimum; SD = standard deviation; T1 = time 1; T2 = time 2; T3 = time 3.
1.2. Procedures
We selected participants who consented to complete in-person clinical interviews to determine their MDD and GAD diagnostic status at T1 and T3. Participants completed in-person clinical interviews at T1, T2, and T3 and a self-report measure of multiple dimensions of sleep quality at T2. Global sleep quality was not assessed at T1 and T3.
1.3. Measures
1.3.1. Major depressive disorder
MDD severity was measured using the Diagnostic and Statistical Manual–Third Edition–Revised (DSM-III-R)–aligned Composite International Diagnostic Interview–Short Form (CIDI-SF) (Kessler et al., 1998). Participants reported if they experienced depression symptoms (7-item; i.e., loss of interest in pleasurable activities, anhedonia, depressed mood, appetite changes, fatigue, suicidal ideation, sleep disturbance) in the past 12months by answering ‘Yes’ (coded as ‘1’) or ‘No’ (coded as ‘0’) to each symptom. The MDD symptom severity scale score can thus range from 0 to 7. The CIDI-SF has exhibited good internal consistency (Cronbach’s α = 0.94 for both T1 and T3 in this study), strong retest reliability, excellent sensitivity (93.9%), and specificity (89.6%) (Wittchen, 1994).
1.3.2. Generalized anxiety disorder
GAD severity was also assessed using the DSM-III-R-consistent CIDI-SF (Kessler et al., 1998). Participants recorded the degree to which they experienced symptoms resulting from their worries for most days in the past year (10-item; i.e., restlessness, feeling keyed up, irritability, trouble sleeping, difficulties focusing, fatigue, muscle tension). The GAD symptom severity score can therefore range from 0 to 10. For GAD, the CIDI-SF has demonstrated good internal consistency (α = 0.80 at T1 and .83 at T3 in this study), strong retest reliability, and excellent sensitivity (99.8%) and specificity (89.6%) (Abel & Borkovec, 1995; Wittchen, 1994).
1.3.3. Sleep quality
Past-month sleep quality and disturbances were assessed with the 19-item Pittsburgh Sleep Quality Index (PSQI) self-report (Buysse et al., 1989). The PSQI comprises seven components. Subjective sleep quality was assessed with a 1-item overall rating of participants’ subjective appraisal of sleep quality (1 = very bad to 4 = very good). Sleep latency (2-item; e.g., “During the past month, how long (in minutes) has it taken you to fall asleep at night?”) assessed the average time one takes to fall asleep. Sleep duration (1-item) measured the average number of hours one actually sleeps each night. Habitual sleep efficiency (3-item) assessed the percentage of time in bed that was spent actually sleeping (e.g., time participants went to bed and woke up). Sleep disturbances (9-item) assessed the frequency with which various scenarios interrupted sleep (i. e., coughing and snoring, bad dreams, pain) (1 = Three or more times per week to 4 = Not during the past month). Use of sleeping medication (1-item) assessed how often one consumed prescribed or “over-the-counter” medication to assist with sleep (1 = Three or more times per week to 4 = Not during the past month). Daytime dysfunction (2-item) inquired on the extent of difficulty to stay awake (1 = A very big problem to 4 = No problem at all). Most items were rated on a 0–3 Likert scale and summed to give a global PSQI score, ranging from 0 to 21. Higher scores indicate better global sleep quality. The PSQI has shown good internal consistency for the seven components (α = 0.83) and 19 individual items (α = 0.83), high retest reliability (r = 0.85), convergent validity, and discriminant validity (Buysse et al., 1989). In this study, the PSQI had good internal consistency (α = 0.70).
2. Data analyses
The R (Version 4.1.0) and RStudio (Version 1.4.1717) (R Core Team, 2021) software were used for data analyses. As part of a series of pre-processing steps, the raw values of the sleep quality and mental health symptom severity variables were inspected to detect highly skewed or kurtotic values. Most of the study variables had acceptable values of skewness ≤ ± 3 and kurtosis of ≤ ± 7 (Field, 2009) (refer to Table 2, which shows the descriptive statistics of all study variables). To handle missing data (9.512% of the entire dataset), we used full information maximum likelihood (FIML). FIML is an optimal approach to reduce bias in parameter estimates based on simulation studies, given the present data was likely to be missing at random (Lee & Shi, 2021; Savalei & Rhemtulla, 2012). Moreover, the Little’s Missing Completely At Random Test (MCAR) was not statistically significant (χ2 (df = 487) = 39.100, p = .464). A series of longitudinal mediation structural equation models with lavaan R package was conducted to determine if T1 GAD substantially predicted T3 MDD severity (or vice versa) through poorer global sleep quality. All models included MDD and GAD at T1 and T2 as covariates. For example, if T3 GAD was the outcome, we adjusted for GAD at T1 and T2. Maximum likelihood with robust estimators were used to manage any univariate or multivariate non-normality in the data distribution (Rhemtulla et al., 2012). Model fit was determined using the significance of the χ2 statistic. Nonetheless, as the χ2 statistic tends to be statistically significant with a large sample size despite a negligible degree of misfit (Bollen & Stine, 1992), other practical fit indices were used to evaluate model fit: confirmatory factor index (CFI; CFI >0.95); root mean squared error of approximation (RMSEA; RMSEA <0.060); and standardized root mean square residual (SRMR; SRMR <0.080) (Maydeu-Olivares, 2017).
Table 2.
Descriptive Statistics of Sociodemographic and Study Variables of Interest.
| M | (SD) | Min | Max | Skewness | Kurtosis | |
|---|---|---|---|---|---|---|
| T1 Age | 45.616 | (11.408) | 20 | 74 | 0.236 | −0.703 |
| T2 Age | 54.544 | (11.352) | 30 | 84 | 0.238 | −0.704 |
| T3 Age | 63.641 | (11.350) | 39 | 93 | 0.240 | −0.706 |
| Gender | ||||||
| Men, n (%) | 1477 | (44.839) | – | – | 4.287 | 38.768 |
| Women, n (%) | 1799 | (54.614) | – | – | – | – |
| Declined to disclose, n (%) | 18 | (0.546) | – | – | – | – |
| Ethnicity | ||||||
| White, n (%) | 3061 | (92.927) | – | – | 5.374 | 29.892 |
| African American, n (%) | 107 | (3.248) | – | – | – | – |
| Native American, n (%) | 11 | (0.334) | – | – | – | – |
| Asian or Pacific Islander, n (%) | 15 | (0.455) | – | – | – | – |
| Other, n (%) | 41 | (1.245) | – | – | – | – |
| Multiracial, n (%) | 23 | (0.699) | – | – | – | – |
| Declined to disclose or missing, n (%) | 36 | (1.093) | – | – | – | – |
| T2 PSQI Subjective sleep quality | 0.963 | (0.674) | 0 | 3 | 0.475 | 0.569 |
| T2 PSQI Sleep latency | 0.949 | (1.189) | 0 | 8 | 2.776 | 12.846 |
| T2 PSQI Sleep duration | 0.792 | (0.746) | 0 | 3 | 0.785 | 0.486 |
| T2 PSQI Habitual sleep efficiency | 0.558 | (0.969) | 0 | 3 | 1.601 | 1.206 |
| T2 PSQI Sleep disturbances | 1.299 | (0.641) | 0 | 8 | 2.876 | 25.301 |
| T2 PSQI Daytime dysfunction | 0.782 | (0.663) | 0 | 3 | 0.499 | 0.201 |
| T1 MDD Loss of interest | 0.164 | (0.370) | 0 | 1 | 1.815 | 1.293 |
| T1 MDD Fatigue | 0.123 | (0.328) | 0 | 1 | 2.295 | 3.270 |
| T1 MDD Appetite change | 0.072 | (0.258) | 0 | 1 | 3.320 | 9.027 |
| T1 MDD Sleep problems | 0.097 | (0.296) | 0 | 1 | 2.719 | 5.396 |
| T1 MDD Focusing issues | 0.120 | (0.325) | 0 | 1 | 2.334 | 3.451 |
| T1 MDD Depressed mood | 0.088 | (0.284) | 0 | 1 | 2.900 | 6.411 |
| T1 MDD Thoughts of death | 0.078 | (0.268) | 0 | 1 | 3.145 | 7.895 |
| M | (SD) | Min | Max | Skewness | Kurtosis | |
| T2 MDD Loss of interest | 0.140 | (0.347) | 0 | 1 | 2.075 | 2.305 |
| T2 MDD Fatigue | 0.108 | (0.311) | 0 | 1 | 2.518 | 4.344 |
| T2 MDD Appetite change | 0.064 | (0.244) | 0 | 1 | 3.570 | 10.745 |
| T2 MDD Sleep problems | 0.086 | (0.280) | 0 | 1 | 2.961 | 6.769 |
| T2 MDD Focusing issues | 0.100 | (0.299) | 0 | 1 | 2.673 | 5.148 |
| T2 MDD Depressed mood | 0.074 | (0.262) | 0 | 1 | 3.243 | 8.518 |
| T2 MDD Thoughts of death | 0.069 | (0.254) | 0 | 1 | 3.393 | 9.514 |
| T3 MDD Loss of interest | 0.160 | (0.367) | 0 | 1 | 1.854 | 1.438 |
| T3 MDD Fatigue | 0.114 | (0.318) | 0 | 1 | 2.421 | 3.862 |
| T3 MDD Appetite change | 0.068 | (0.252) | 0 | 1 | 3.430 | 9.771 |
| T3 MDD Sleep problems | 0.086 | (0.281) | 0 | 1 | 2.947 | 6.687 |
| T3 MDD Focusing issues | 0.100 | (0.300) | 0 | 1 | 2.662 | 5.088 |
| T3 MDD Depressed mood | 0.075 | (0.263) | 0 | 1 | 3.226 | 8.410 |
| T3 MDD Thoughts of death | 0.073 | (0.260) | 0 | 1 | 3.277 | 8.740 |
| T1 GAD Restlessness | 0.815 | (1.327) | 0 | 4 | 1.197 | −0.261 |
| T1 GAD Irritability | 0.780 | (1.274) | 0 | 4 | 1.208 | −0.233 |
| T1 GAD Sleep issues | 0.837 | (1.384) | 0 | 4 | 1.262 | −0.077 |
| T1 GAD Concentration problems | 0.863 | (1.395) | 0 | 4 | 1.164 | −0.362 |
| T1 GAD Low energy | 0.845 | (1.394) | 0 | 4 | 1.255 | −0.096 |
| T1 GAD Muscle tension | 0.896 | (1.477) | 0 | 4 | 1.234 | −0.208 |
| T2 GAD Restlessness | 0.701 | (1.270) | 0 | 4 | 1.439 | 0.395 |
| T2 GAD Irritability | 0.684 | (1.246) | 0 | 4 | 1.461 | 0.473 |
| T2 GAD Sleep issues | 0.685 | (1.281) | 0 | 4 | 1.578 | 0.891 |
| T2 GAD Concentration problems | 0.715 | (1.304) | 0 | 4 | 1.465 | 0.475 |
| T2 GAD Low energy | 0.694 | (1.293) | 0 | 4 | 1.566 | 0.841 |
| T2 GAD Muscle tension | 0.744 | (1.386) | 0 | 4 | 1.529 | 0.636 |
| T3 GAD Restlessness | 0.688 | (1.267) | 0 | 4 | 1.497 | 0.592 |
| T3 GAD Irritability | 0.681 | (1.255) | 0 | 4 | 1.501 | 0.614 |
| T3 GAD Sleep issues | 0.654 | (1.243) | 0 | 4 | 1.644 | 1.142 |
| T3 GAD Concentration problems | 0.703 | (1.299) | 0 | 4 | 1.500 | 0.582 |
| T3 GAD Low energy | 0.658 | (1.251) | 0 | 4 | 1.648 | 1.159 |
| T3 GAD Muscle tension | 0.759 | (1.413) | 0 | 4 | 1.512 | 0.552 |
| M | (SD) | Min | Max | Skewness | Kurtosis | |
| T1 Chronic sleep problems, n (%) | 332 | (10.079) | – | – | −2.651 | 5.029 |
| T2 Chronic sleep problems, n (%) | 295 | (8.956) | – | – | −2.874 | 6.259 |
| T3 Chronic sleep problems, n (%) | 338 | (10.261) | – | – | −2.618 | 4.855 |
| T1 Number of chronic conditions | 2.133 | (2.290) | 0 | 21 | 1.707 | 4.733 |
| T2 Number of chronic conditions | 2.010 | (2.338) | 0 | 30 | 2.366 | 12.731 |
| T3 Number of chronic conditions | 2.638 | (3.107) | 0 | 20 | 1.677 | 3.440 |
| T1 Number of medications | 0.000 | (0.000) | 0 | 0 | – | – |
| T2 Number of medications | 1.243 | (1.562) | 0 | 12 | 1.749 | 4.910 |
| T3 Number of medications | 1.214 | (1.483) | 0 | 9 | 1.351 | 1.817 |
| T1 Regular physical exercise, n (%) | 619 | (18.792) | – | – | −1.597 | 0.551 |
| T2 Regular physical exercise, n (%) | 767 | (23.285) | – | – | −1.264 | −0.403 |
| T3 Regular physical exercise, n (%) | 965 | (29.296) | – | – | −0.909 | −1.173 |
| T1 Household total income ($) | 76200.516 | (64898.144) | 0 | 300000 | 1.377 | 1.741 |
| T2 Household total income ($) | 59698.427 | (55561.518) | 0 | 298750 | 1.185 | 1.563 |
| T3 Household total income ($) | 58966.910 | (64082.607) | 0 | 299250 | 1.249 | 1.208 |
| T1 Current financial situation | 6.008 | (2.462) | 0 | 10 | −0.848 | 0.255 |
| T2 Current financial situation | 5.709 | (2.961) | 0 | 10 | −0.773 | −0.470 |
| T3 Current financial situation | 5.470 | (3.308) | 0 | 10 | −0.582 | −1.001 |
Note. GAD = generalized anxiety disorder; MDD = major depressive disorder; Max = maximum; Min = minimum; PSQI = Pittsburgh sleep quality index.
Note. The current financial situation was measured on a scale of 0 = the worst possible financial crisis to 10 = the best possible financial situation.
To conduct mediation analyses, we used a product-of-coefficients approach. First, a direct effect (c path) was signified by a significant connection between the predictor (T1 MDD or GAD severity) and outcome (T3 MDD or GAD severity) (c’ path). Second, we determined if the predictor (T1 MDD or GAD severity) was significantly related to the mediator (T2 GSQ) (a path) and if the mediator (T2 GSQ) was notably associated with the outcome (T3 MDD or GAD severity) (b path). A mediation effect would be denoted by a statistically significant multiplication between paths a and b (indirect effect, c = a*b), above and beyond the direct effect. Further, as part of best practices to obtain less unbiased and more efficient, stable, and reliable parameter estimates, particularly in the context of multivariate non-normality, a bootstrapping approach was used with 10,000 iterations (Bollen & Stine, 1992; Yuan et al., 2007). In addition, we computed mediation effect sizes that represented the percentage of the variance of the outcome that was contributed jointly by the predictor and mediator after adjusting for any spurious associations produced by the temporal ordering of the variables (Lachowicz et al., 2018). In addition, effect sizes for all regression parameter estimates were calculated using the formula, d = 2t/√(df), wherein t referred to the t-statistic of the respective parameter and df the degrees of freedom of the error term (Dunst et al., 2004). Moreover, based on the literature, we conducted a sensitivity analysis to test if any observed statistically significant effects remained after controlling for these potential covariates: age (Crowley, 2011), gender (Zeng et al., 2020), presence of chronic sleep problems, past year number of chronic health conditions (i.e., asthma, bronchitis, or emphysema, tuberculosis, other lung problems, joint or bone diseases, sciatica, lumbago, or backache, skin trouble, thyroid disease, hay fever, stomach trouble, urinary or bladder problem, constipation issues, gall bladder trouble, foot problems, varicose veins, AIDS or HIV, lupus or autoimmune disorder, gum or mouth problems, dental problems, hypertension, alcohol or drugs, migraine, diabetes or high blood sugar, neurological disease, stroke, ulcer, hernia, piles or hemorrhoids, or swallowing problems) (Campanini et al., 2021), past year number of prescribed medication consumed (hypertension, diabetes, cholesterol, heart condition, lung problems, ulcer, arthritis, hormone therapy, birth control pills, headaches, anxiety or depression, pain) (Campanini et al., 2021), regular physical exercise (Xie et al., 2021), household total income, and current financial situation (0 = the worst possible financial crisis to 10 = the best possible financial situation) (Grandner et al., 2016). Furthermore, we controlled for T1 and T2 MDD when T3 MDD severity was the outcome and controlled for T1 and T2 GAD when predicting T3 GAD. Table 2 details descriptive statistics of all study variables. Readers can access the analytic data script from OSF (https://osf.io/qct2y/) and the dataset from the ICPSR repository (https://www.icpsr.umich.edu/web/ICPSR/series/203).
3. Results
Hypothesis 1.
T1 GAD → T3 MDD mediated by T2 Global Sleep Quality.
First, we examined if the T1 GAD–T3 MDD relation would be mediated by T2 global sleep quality over and above T1 and T2 MDD severity. Based on the practical fit indices, this mediation model showed good fit (χ2 (df = 487) = 1080.834, p < .001, CFI = .970, TLI = .968, RMSEA = .036, SRMR = .059). Supporting Hypothesis 1, higher T1 GAD symptom severity significantly predicted T2 global sleep quality (β = −0.078, SE = 0.015, z = −5.293, p < .001, d = −0.480), and less T2 global sleep quality was significantly related to higher T3 MDD severity (β = −0.183, SE = 0.030, z = −3.039, p < .001, d = −0.275). Simultaneously, the indirect effect (T1 GAD → T2 global sleep quality → T3 MDD) was significant (β = 0.014, SE = 0.006, z = 2.478, p = .013, d = 0.225). T2 global sleep quality mediated 41.340% of the T1 GAD–T3 MDD association. Fig. 1 presents the full SEM model of Hypothesis 1.
Fig. 1. Longitudinal Structural Equation Mediation Modeling of GAD Severity Predicting MDD Severity.
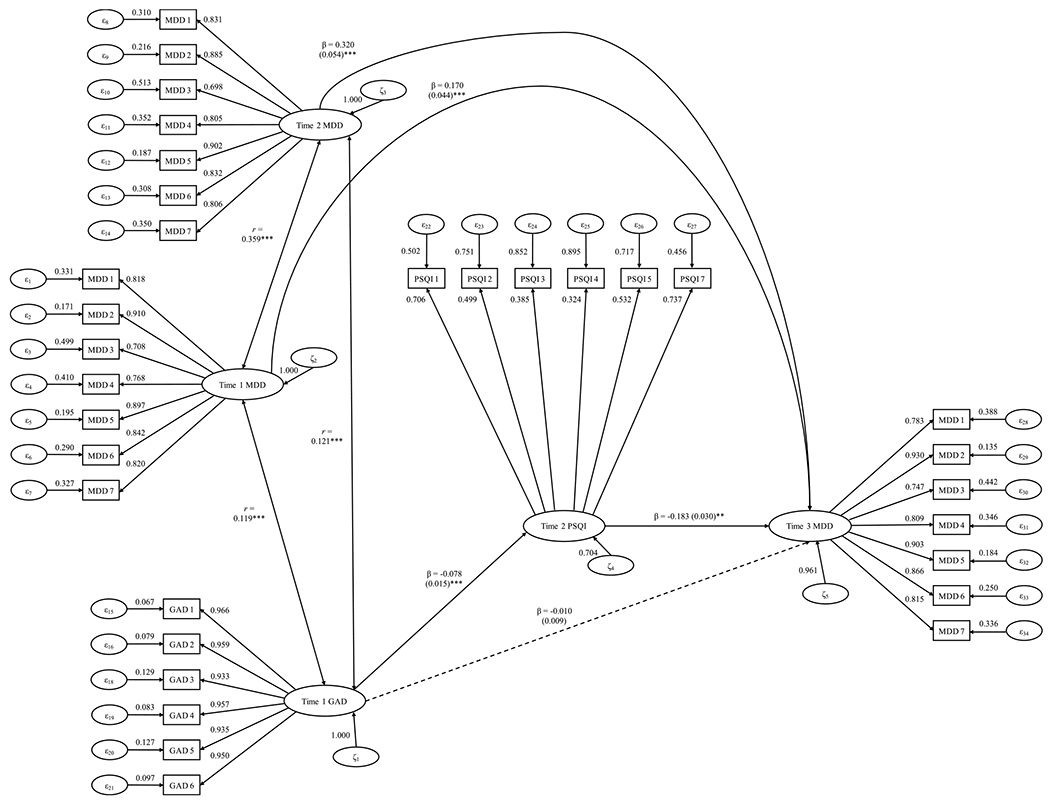
Note. * ** p < .001; * p < .05. GAD = generalized anxiety disorder; MDD = major depressive disorder. This figure shows the unstandardized regression path coefficients and their standard error in parentheses. Bold lines indicate statistically significant effects, whereas dotted lines denote parameter estimates that were not statistically significant.
Moreover, the indirect effect remained significant after controlling for these covariates: age (d = 0.208), gender (d = 0.208), past-12-month chronic sleep problems (d = 0.210), number of chronic health conditions (d = 0.210), number of medications taken (d = 0.213), presence of coregular physical exercise (d = 0.206), total household income (d = 0.206), and current financial situation (d = 0.209) (ps = .012 to .013). Overall, Hypothesis 1 was fully supported.
Hypothesis 2.
T1 MDD → T3 GAD mediated by T2 Global Sleep Quality.
Next, we examined if T2 global sleep quality would mediate the T1 MDD–T3 GAD relation above and beyond T1 and T2 GAD severity. Based on the practical fit indices, this mediation model showed good fit (χ2 (df = 426) = 625.192, p < .001, CFI = .993, TLI = .992, RMSEA = .022, SRMR = .042). Higher T1 MDD symptom severity was significantly associated with lower T2 global sleep quality (β = −0.606, SE = 0.105, z= −5.789, p < .001, d = −0.561), and reduced T2 global sleep quality was significantly linked to greater T3 MDD severity (β = −0.214, SE = 0.109, z= −1.960, p = .050, d = −0.190). Simultaneously, the indirect effect (T1 MDD → T2 global sleep quality → T3 GAD) was statistically significant (β = 0.130, SE = 0.062, z = 2.104, p = .035, d = 0.204). T2 Global Sleep Quality mediated 10.819% of the T1 MDD–T3 GAD relation. Therefore, Hypothesis 2 was supported (see Fig. 2).1
Fig. 2. Longitudinal Structural Equation Mediation Modeling of MDD Severity Predicting GAD Severity.
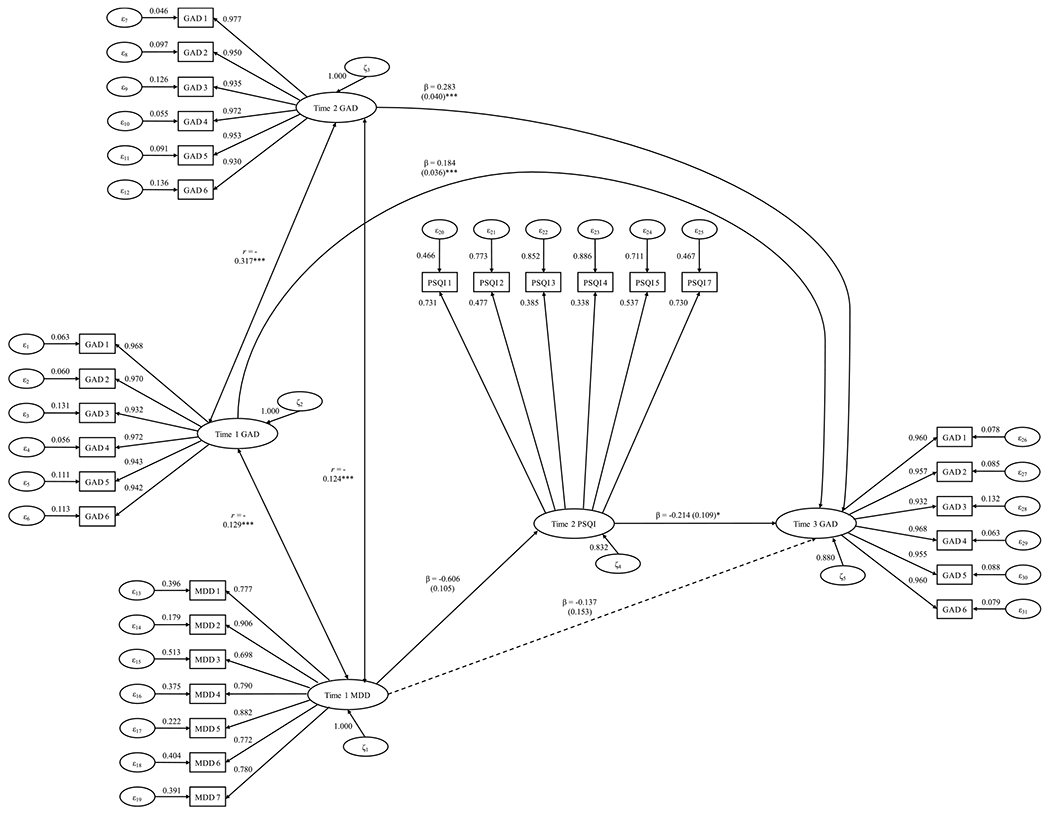
Note. * ** p < .001; * p < .05. GAD = generalized anxiety disorder; MDD = major depressive disorder. This figure shows the unstandardized regression path coefficients and their standard error in parentheses. Bold lines indicate statistically significant effects, whereas dotted lines denote parameter estimates that were not statistically significant.
Furthermore, the indirect effect stayed statistically significant after adjusting for these covariates: age (d = 0.183), gender (d = 0.197), chronic sleep issues (d = 0.180), number of medications consumed (d = 0.190), regular engagement in physical exercise (d = 0.187), total household income (d = 0.184), and current financial situation (d = 0.178). Collectively, the results were fully consistent with Hypothesis 2.
4. Discussion
To our knowledge, this is the first study to test if poorer global subjective sleep quality mediated the pathway of GAD predicting MDD severity 18 years later and vice versa. We found that poorer global sleep quality mediated the relation between T1 MDD and T3 GAD (controlling for T1 and T2 GAD). Likewise, worse global sleep quality mediated the T1 GAD-T3 MDD association. Specifically, T2 global sleep quality accounted for about 41% of the T1 GAD–T3 MDD association and approximately 11% of the T1 MDD–T3 GAD relation. Collectively, our results thus lend support to the transdiagnostic theory of sleep (Harvey, 2008). We provide potential explanations for this pattern of findings.
Why did T1 MDD and GAD predict T2 global subjective sleep quality? One plausible explanation is that sleep issues and MDD and GAD have similar underlying risk factors, such as repetitive negative thinking patterns. Consistent with Riemann’s hyperarousal model, insomnia could exacerbate a cycle of pre-sleep negative perseverative cognitions over time, some of which are often implicated in depression and anxiety disorder-prone individuals (e.g., excessive worry rumination, limiting core beliefs). Repetitive negative thinking patterns may hinder the ability to achieve good quality sleep over time (Jansson & Linton, 2007; Mazzer et al., 2019; Norell-Clarke et al., 2014; Riemann et al., 2010).
Findings that greater MDD and GAD symptom severity predicted lower global sleep quality might also be partly explained by scar theories. These theories propose that higher MDD and GAD symptoms may create a “scarring effect” over time by predisposing individuals to poorer sleep quality through various deficient health behaviors, such as unhealthy diet and excessive technology use. Plausibly, those exhibiting elevated anxiety and depressive symptoms predispose themselves to future sleep issues by making poor diet choices (Zhao et al., 2020). There is a growing body of evidence for links between more unhealthy dietary choices and increased anxiety and depressive symptoms in the long term (Daneshzad et al., 2020; Tan et al., 2016). Prolonged Internet, smartphone, and digital tablet overuse (e.g., excessive social media consumption) amongst anxiety and depression-prone individuals may influence the link between T1 MDD and GAD and T2 subjective sleep quality (Kaur et al., 2021; Lam, 2014). These processes could unfold by affecting sleep architecture, altering melatonin levels via excessive exposure to bright lights during bedtime, and inducing cognitive, emotional, and physiological arousal (Dinis & Bragança, 2018). These conjectures warrant testing by future studies.
Why did T2 global subjective sleep quality predict T3 MDD and GAD? Suboptimal stress and coping strategies may mediate the association between poor sleep quality and MDD or GAD 9 years later. Prolonged stress reactivity has been linked to sleep problems, possibly due to fatigue and other physiological markers associated with high stress reactivity (e.g., higher resting heart rate and blood pressure) (Herr et al., 2018). As a result, sleep issues may, over time, increase vulnerability to stress, which has already been identified as a significant risk factor for anxiety and depressive disorder-prone individuals (Michl, McLaughlin, Shepherd, & Nolen-Hoeksema, 2013; Zhang, Yan, Shum, & Deng, 2020). Our findings also extend a recent study that showed poor sleep quality was associated with a larger rise in somatic symptoms of depression (e. g., fatigue) among college students (and vice versa) (Shim et al., 2019). This pattern may be attributed to daytime dysfunction reducing serotonergic and dopaminergic transmission critical for regulating sleep-wake cycle biological rhythms, and the adverse effects of poor sleep on cognitive functions (Harvey et al., 2011; Waller et al., 2016). Future research on sleep-deprived or sleep behavior-disordered individuals could provide insight into the role of dysregulated dopamine on long-term anxiety and depressive symptoms. Specifically, the impact on reward brain circuitry (e.g., nucleus accumbens, orbitofrontal cortex, striatum) may have direct effects on reward processing such that sleep-disturbed individuals demonstrate depression-like behaviors (i.e., helplessness, anhedonia, inability to concentrate) (Auerbach et al., 2014; Blake et al., 2018; Martin-Soelch, 2009). These hypotheses merit empirical testing.
Overall, our findings that T2 global sleep quality mediates the prospective 18-year MDD-GAD bi-directional relation is consistent with some prior longitudinal research. For instance, at least four studies have shown that sleep duration, disturbance, and associated insomnia markers were reciprocally related to depression and anxiety disorder severity in diverse adolescent, pregnant women, and community-dwelling samples (Geng et al., 2018; Goldstone et al., 2020; Liu et al., 2020; van der Zwan et al., 2017). The current paper builds upon these studies by examining suboptimal sleep as a mechanism of the longitudinal MDD-GAD comorbidity.
Our study has some limitations. First, we did not use any objective measures of sleep quality (e.g., electroencephalogram, polysomnography). Future studies should consider utilizing multiple sleep latency tests to capture better psychobiological sleep profiles (Brand et al., 2014; Plante et al., 2017; Zhu et al., 2020). Also, given the use of DSM-III-R-defined CIDI-SF interviews, future research can determine if a similar pattern of findings is replicated using DSM-5 measures. In addition, to increase generalizability, it would be optimal for upcoming studies to recruit diverse samples in terms of socioeconomic status and ethnicity, given the sample homogeneity in this study. Indeed, studies suggest racial and ethnic disparities in sleep health (e.g., lower sleep quality and duration among African Americans than other racial groups) (Johnson et al., 2019). Finally, as sleep quality did not fully mediate the 18-year GAD and MDD relations, unexamined third variables may play a mechanistic role in this developmental association, such as coping strategies (Marr et al., 2022) and merit attention.
Nonetheless, our study has several strengths. For instance, the longitudinal nature of our analysis provides more insight into the temporal relation between sleep issues and two common psychiatric disorders. Additionally, the dataset included a wide age range of participants. As sleep issues influence people throughout their lifespan, our research sufficiently captures how sleep difficulties may precede or be a consequence of mental disorders across various developmental stages. Last, since the findings remained similar after removing sleep items from the analyses and adjusting for baseline and mid-time-point MDD and GAD, more inferior sleep quality could be a robust predictor and consequence of these common mental health problems.
If future studies replicate our pattern of findings, some clinical implications merit consideration. For instance, therapies that address sleep issues and psychiatric disorders, such as cognitive-behavioral therapy for insomnia (CBTI), appear to have promising outcomes for improving symptoms. Examples include the effectiveness of CBTI in changing maladaptive thinking and somatic hyperarousal, which are characteristic of both insomnia, depression, and anxiety disorders (Kalmbach et al., 2019). Progressive muscle relaxation and applied relaxation could be beneficial in reducing somatic tension, worries, and related depression and anxiety symptoms, to facilitate better sleep as part of CBTI or as effective standalone treatments (Neuendorf et al., 2015). Another type of therapy that employs this tactic of addressing both sleep and psychiatric issues is mindfulness-based cognitive therapy (MBCT). Research has consistently shown that MBCT effectively reduces anxiety and depressive symptoms and improves subjective sleep quality (Britton et al., 2010). The treatment mechanisms have included modifications in pre-sleep arousal, such as decrements in unhelpful avoidance behaviors and classically conditioned anxious arousal or pathological worry with the bed (Blake et al., 2017). Further, several preliminary studies have demonstrated that music therapy may elicit psychophysiological responses to musical pitch and rhythm, resulting in reductions in depressive symptoms alongside sleep issues (Chan et al., 2010; Chan et al., 2009; Wang et al., 2014).
In closing, our findings further refine the longitudinal between-subject relationship between MDD and GAD across 18 years. Global subjective sleep quality at T2 mediated the T1 MDD–T3 GAD relation and vice versa. Future studies should consider utilizing objective measures of sleep quality and DSM-5 measures and recruiting culturally diverse samples.
Acknowledgements
The data used in this publication were made available by the Data Archive on the University of Wisconsin - Madison Institute on Aging, 1300 University Avenue, 2245 MSC, Madison, Wisconsin 53706-1532. Since 1995 the MIDUS study has been funded by the following: John D. and Catherine T. MacArthur Foundation Research Network; National Institute on Aging (P01-AG020166); National Institute on Aging (U19-AG051426). The original investigators and funding agency are not responsible for the analyses or interpretations presented here.
Footnotes
For both Hypotheses 1 and 2, we determined that the same pattern of findings remained after removing the sleep disturbance symptom items from the MDD and GAD measures in the longitudinal SEM mediation analyses (refer to p. 1 in the online supplementary materials; OSM).
Data availability
We have shared the link to our code and output at the Attach File step.
References
- Abel JL, & Borkovec TD (1995). Generalizability of DSM-III-R generalized anxiety disorders to proposed DSM-IV criteria and cross-validation of proposed changes. Journal of Anxiety Disorders, 9(4), 303–315. 10.1016/0887-6185(95)00011-C [DOI] [Google Scholar]
- Auerbach RP, Admon R, & Pizzagalli DA (2014). Adolescent depression: Stress and reward dysfunction. Harvard Review of Psychiatry, 22(3), 139–148. 10.1097/HRP.0000000000000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M, Schwartz O, Waloszek JM, Raniti M, Simmons JG, Murray G, & Allen NB (2017). The SENSE study: Treatment mechanisms of a cognitive behavioral and mindfulness-based group sleep improvement intervention for at-risk adolescents. Sleep, 40(6). 10.1093/sleep/zsx061 [DOI] [PubMed] [Google Scholar]
- Blake MJ, Trinder JA, & Allen NB (2018). Mechanisms underlying the association between insomnia, anxiety, and depression in adolescence: Implications for behavioral sleep interventions. Clinical Psychology Review, 63, 25–40. 10.1016/j.cpr.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Bollen KA, & Stine RA (1992). Bootstrapping goodness-of-fit measures in structural equation models. Sociological Methods & Research, 21(2), 205–229. 10.1177/0049124192021002004 [DOI] [Google Scholar]
- Brand S, Kalak N, Gerber M, Kirov R, Pühse U, & Holsboer-Trachsler E (2014). High self-perceived exercise exertion before bedtime is associated with greater objectively assessed sleep efficiency. Sleep Medicine, 15(9), 1031–1036. 10.1016/j.sleep.2014.05.016 [DOI] [PubMed] [Google Scholar]
- Brim OG, Baltes PB, Bumpass LL, Cleary PD, Featherman DL, Hazzard WR, & Shweder RA (2019). Midlife in the United States (MIDUS 1). 1995-1996 Inter-University Consortium for Political and Social Research [distributor]. 10.3886/ICPSR02760.v18 [DOI] [Google Scholar]
- Britton WB, Haynes PL, Fridel KW, & Bootzin RR (2010). Polysomnographic and subjective profiles of sleep continuity before and after Mindfulness-Based Cognitive Therapy in partially remitted depression. Psychosomatic Medicine, 72(6), 539–548. 10.1097/PSY.0b013e3181dc1bad [DOI] [PubMed] [Google Scholar]
- Butnoriene J, Bunevicius A, Saudargiene A, Nemeroff CB, Norkus A, Ciceniene V, & Bunevicius R (2015). Metabolic syndrome, major depression, generalized anxiety disorder, and ten-year all-cause and cardiovascular mortality in middle aged and elderly patients. International Journal of Cardiology, 190, 360–366. 10.1016/j.ijcard.2015.04.122 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, & Rössler W (2008). Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep, 31(4), 473–480. 10.1093/sleep/31.4.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Campanini MZ, Gonzalez AD, de Andrade SM, Girotto E, Cabrera MAS, Cavero-Redondo I, & Mesas AE (2021). The association of continuous-use medications and sleep parameters in a sample of working adults. Sleep and Breathing, 25(4), 2205–2212. 10.1007/s11325-021-02343-x [DOI] [PubMed] [Google Scholar]
- Chalmers T, Hickey BA, Newton P, Lin C-T, Sibbritt D, McLachlan CS, & Lal S (2022). Associations between sleep quality and heart rate variability: Implications for a biological model of stress detection using wearable technology. International Journal of Environmental Research and Public Health, 19(9). 10.3390/ijerph19095770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MF, Chan EA, & Mok E (2010). Effects of music on depression and sleep quality in elderly people: A randomised controlled trial. Complementary Therapies in Medicine, 18(3–4), 150–159. 10.1016/j.ctim.2010.02.004 [DOI] [PubMed] [Google Scholar]
- Chan MF, Chan EA, Mok E, & Kwan Tse FY (2009). Effect of music on depression levels and physiological responses in community-based older adults. International Journal of Mental Health Nursing, 18(4), 285–294. 10.1111/j.1447-0349.2009.00614.x [DOI] [PubMed] [Google Scholar]
- Cho HJ, Seeman TE, Kiefe CI, Lauderdale DS, & Irwin MR (2015). Sleep disturbance and longitudinal risk of inflammation: Moderating influences of social integration and social isolation in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Brain, Behavior, and Immunity, 46, 319–326. 10.1016/j.bbi.2015.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley K (2011). Sleep and sleep disorders in older adults. Neuropsychology Review, 21 (1), 41–53. 10.1007/s11065-010-9154-6 [DOI] [PubMed] [Google Scholar]
- Daneshzad E, Keshavarz S-A, Qorbani M, Larijani B, & Azadbakht L (2020). Association between a low-carbohydrate diet and sleep status, depression, anxiety, and stress score. Journal of the Science of Food and Agriculture, 100(7), 2946–2952. 10.1002/jsfa.10322 [DOI] [PubMed] [Google Scholar]
- Dinis J, & Bragança M (2018). Quality of sleep and depression in college students: A systematic review. Sleep Science, 11(4), 290–301. 10.5935/1984-0063.20180045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds KL, Miller CB, Kyle SD, Marshall NS, & Gordon CJ (2017). Heart rate variability in insomnia patients: A critical review of the literature. Sleep Medicine Reviews, 33, 88–100. 10.1016/j.smrv.2016.06.004 [DOI] [PubMed] [Google Scholar]
- Dunst CJ, Hamby DW, & Trivette CM (2004). Guidelines for calculating effect sizes for practice-based research syntheses. Centerscope, 3(1), 1–10. [Google Scholar]
- Field AP (2009). Discovering statistics using SPSS. SAGE Publications. [Google Scholar]
- Geng F, Liu X, Liang Y, Shi X, Chen S, & Fan F (2018). Prospective associations between sleep problems and subtypes of anxiety symptoms among disaster-exposed adolescents. Sleep Medicine, 50, 7–13. 10.1016/j.sleep.2018.05.017 [DOI] [PubMed] [Google Scholar]
- Goldstone A, Javitz HS, Claudatos SA, Buysse DJ, Hasler BP, de Zambotti M, & Baker FC (2020). Sleep disturbance predicts depression symptoms in early adolescence: Initial findings from the adolescent brain cognitive development study. Journal of Adolescent Health, 66(5), 567–574. 10.1016/j.jadohealth.2019.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Williams NJ, Knutson KL, Roberts D, & Jean-Louis G (2016). Sleep disparity, race/ethnicity, and socioeconomic position. Sleep Medicine, 18, 7–18. 10.1016/j.sleep.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AM, Caspi A, Eley TC, Moffitt TE, Oconnor TG, & Poulton R (2005). Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. Journal of Abnormal Child Psychology, 33(2), 157–163. 10.1007/s10802-005-1824-0 [DOI] [PubMed] [Google Scholar]
- Grima NA, Bei B, & Mansfield D (2019). Insomnia theory and assessment. Australian Journal of General Practice, 48(4), 193–197. 10.31128/ajgp-12-18-4780 [DOI] [PubMed] [Google Scholar]
- Harvey AG (2008). Insomnia, psychiatric disorders, and the transdiagnostic perspective. Current Directions in Psychological Science, 17(5), 299–303. 10.1111/j.1467-8721.2008.00594.x [DOI] [Google Scholar]
- Harvey AG, Murray G, Chandler RA, & Soehner A (2011). Sleep disturbance as transdiagnostic: Consideration of neurobiological mechanisms. Clinical Psychology Review, 31(2), 225–235. 10.1016/j.cpr.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr RM, Barrech A, Riedel N, Gündel H, Angerer P, & Li J (2018). Long-term effectiveness of stress management at work: Effects of the changes in perceived stress reactivity on mental health and sleep problems seven years later. International Journal of Environmental Research and Public Health, 15(2). 10.3390/ijerph15020255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao FH, Chang KJ, Kuo WH, Huang CS, Liu YF, Lai YM, & Chan CL (2013). A longitudinal study of cortisol responses, sleep problems, and psychological well-being as the predictors of changes in depressive symptoms among breast cancer survivors. Psychoneuroendocrinology, 38(3), 356–366. 10.1016/j.psyneuen.2012.06.010 [DOI] [PubMed] [Google Scholar]
- Jackson ML, Sztendur EM, Diamond NT, Byles JE, & Bruck D (2014). Sleep difficulties and the development of depression and anxiety: A longitudinal study of young Australian women. Archives of Women’s Mental Health, 17(3), 189–198. 10.1007/s00737-014-0417-8 [DOI] [PubMed] [Google Scholar]
- Jacobson NC, & Newman MG (2017). Anxiety and depression as bidirectional risk factors for one another: A meta-analysis of longitudinal studies. Psychological Bulletin, 143(11), 1155–1200. 10.1037/bul0000111 [DOI] [PubMed] [Google Scholar]
- Jansson M, & Linton SJ (2007). Psychological mechanisms in the maintenance of insomnia: Arousal, distress, and sleep-related beliefs. Behaviour Research and Therapy, 45(3), 511–521. 10.1016/j.brat.2006.04.003 [DOI] [PubMed] [Google Scholar]
- Jansson-Fröjmark M, & Lindblom K (2008). A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. Journal of Psychosomatic Research, 64(4), 443–449. 10.1016/j.jpsychores.2007.10.016 [DOI] [PubMed] [Google Scholar]
- Johnson DA, Jackson CL, Williams NJ, & Alcantara C (2019). Are sleep patterns influenced by race/ethnicity - A marker of relative advantage or disadvantage? Evidence to date. Nature and Science of Sleep, 11, 79–95. 10.2147/NSS.S169312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach DA, Cheng P, Arnedt JT, Anderson JR, Roth T, Fellman-Couture C, & Drake CL (2019). Treating insomnia improves depression, maladaptive thinking, and hyperarousal in postmenopausal women: Comparing cognitive-behavioral therapy for insomnia (CBTI), sleep restriction therapy, and sleep hygiene education. Sleep Medicine, 55, 124–134. 10.1016/j.sleep.2018.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Dhir A, Alkhalifa AK, & Tandon A (2021). Social media platforms and sleep problems: A systematic literature review, synthesis and framework for future research. Internet Research, 31(4), 1121–1152. 10.1108/intr-04-2020-0187 [DOI] [Google Scholar]
- Kessler RC, Gruber M, Hettema JM, Hwang I, Sampson N, & Yonkers KA (2008a). Co-morbid major depression and generalized anxiety disorders in the National Comorbidity Survey Follow-Up. Psychological Medicine, 38(3), 365–374. 10.1017/S0033291707002012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Heeringa S, Lakoma MD, Petukhova M, Rupp AE, Schoenbaum M, & Zaslavsky AM (2008b). Individual and societal effects of mental disorders on earnings in the United States: results from the national comorbidity survey replication. American Journal of Psychiatry, 165(6), 703–711. 10.1176/appi.ajp.2008.08010126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Wittchen H-U, Abelson JM, Mcgonagle K, Schwarz N, Kendler KS, & Zhao S (1998). Methodological studies of the Composite International Diagnostic Interview (CIDI) in the US national comorbidity survey (NCS). International Journal of Methods in Psychiatric Research, 7(1), 33–55. 10.1002/mpr.33 [DOI] [Google Scholar]
- Kim J-M, Stewart R, Kim S-W, Yang S-J, Shin I, & Yoon J-S (2009). Insomnia, depression, and physical disorders in late life: A 2-year longitudinal community study in Koreans. Sleep, 32(9), 1221–1228. 10.1093/sleep/32.9.1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachowicz MJ, Preacher KJ, & Kelley K (2018). A novel measure of effect size for mediation analysis. Psychological Methods, 23(2), 244–261. 10.1037/met0000165 [DOI] [PubMed] [Google Scholar]
- Lam LT (2014). Internet gaming addiction, problematic use of the internet, and sleep problems: A systematic review. Current Psychiatry Reports, 16(4), 444. 10.1007/s11920-014-0444-1 [DOI] [PubMed] [Google Scholar]
- Lee T, & Shi D (2021). A comparison of full information maximum likelihood and multiple imputation in structural equation modeling with missing data. Psychological Methods, 26(4), 466–485. 10.1037/met0000381 [DOI] [PubMed] [Google Scholar]
- Liu B-P, Wang X-T, Liu Z-Z, Wang Z-Y, An D, Wei Y-X, & Liu X (2020). Depressive symptoms are associated with short and long sleep duration: A longitudinal study of Chinese adolescents. Journal of Affective Disorders, 263, 267–273. 10.1016/j.jad.2019.11.113 [DOI] [PubMed] [Google Scholar]
- Marr NS, Zainal NH, & Newman MG (2022). Focus on and venting of negative emotion mediates the 18-year bi-directional relations between major depressive disorder and generalized anxiety disorder diagnoses. Journal of Affective Disorders, 303, 10–17. 10.1016/j.jad.2022.01.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Soelch C (2009). Is depression associated with dysfunction of the central reward system. Biochemical Society Transactions, 37(1), 313–317. 10.1042/bst0370313 [DOI] [PubMed] [Google Scholar]
- Maydeu-Olivares A (2017). Assessing the size of model misfit in structural equation models. Psychometrika. 10.1007/s11336-016-9552-7 [DOI] [PubMed] [Google Scholar]
- Mazzer K, Boersma K, & Linton SJ (2019). A longitudinal view of rumination, poor sleep and psychological distress in adolescents. Journal of Affective Disorders, 245, 686–696. 10.1016/j.jad.2018.11.053 [DOI] [PubMed] [Google Scholar]
- Michl LC, McLaughlin KA, Shepherd K, & Nolen-Hoeksema S (2013). Rumination as a mechanism linking stressful life events to symptoms of depression and anxiety: Longitudinal evidence in early adolescents and adults. Journal of Abnormal Psychology, 122(2), 339–352. 10.1037/a0031994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morphy H, Dunn KM, Lewis M, Boardman HF, & Croft PR (2007). Epidemiology of insomnia: a longitudinal study in a UK population. Sleep, 30(3), 274–280. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17425223. [PubMed] [Google Scholar]
- Neuendorf R, Wahbeh H, Chamine I, Yu J, Hutchison K, & Oken BS (2015). The effects of mind-body interventions on sleep quality: A systematic review. Evidence-Based Complementary and Alternative Medicine, 2015, Article 902708. 10.1155/2015/902708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norell-Clarke A, Jansson-Fröjmark M, Tillfors M, Harvey AG, & Linton SJ (2014). Cognitive processes and their association with persistence and remission of insomnia: Findings from a longitudinal study in the general population. Behaviour Research and Therapy, 54, 38–48. 10.1016/j.brat.2014.01.002 [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Tang W, Gilchrist MA, Moynihan JA, Pressman EK, & Blackmore ER (2014). Diurnal cortisol patterns and psychiatric symptoms in pregnancy: Short-term longitudinal study. Biological Psychology, 96, 35–41. 10.1016/j.biopsycho.2013.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante DT, Finn LA, Hagen EW, Mignot E, & Peppard PE (2017). Longitudinal associations of hypersomnolence and depression in the Wisconsin Sleep Cohort Study. Journal of Affective Disorders, 207, 197–202. 10.1016/j.jad.2016.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [Google Scholar]
- Regier DA, Rae DS, Narrow WE, Kaelber CT, & Schatzberg AF (1998). Prevalence of anxiety disorders and their comorbidity with mood and addictive disorders. British Journal of Psychiatry, 173(Suppl. 34), 24–28. 10.1192/S0007125000293483 [DOI] [PubMed] [Google Scholar]
- Rhemtulla M, Brosseau-Liard PÉ, & Savalei V (2012). When can categorical variables be treated as continuous? A comparison of robust continuous and categorical SEM estimation methods under suboptimal conditions. Psychological Methods, 17(3), 354–373. 10.1037/a0029315 [DOI] [PubMed] [Google Scholar]
- Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, & Nissen C (2010). The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Medicine Reviews, 14(1), 19–31. 10.1016/j.smrv.2009.04.002 [DOI] [PubMed] [Google Scholar]
- Rosellini AJ, & Brown TA (2011). The NEO Five-Factor Inventory: Latent structure and relationships with dimensions of anxiety and depressive disorders in a large clinical sample. Assessment, 18(1), 27–38. 10.1177/1073191110382848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten S, Vriend C, van der Werf YD, Berendse HW, Weintraub D, & van den Heuvel OA (2017). The bidirectional longitudinal relationship between insomnia, depression and anxiety in patients with early-stage, medication-naïve Parkinson’s disease. Parkinsonism & Related Disorders, 39, 31–36. 10.1016/j.parkreldis.2017.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryff C, Almeida D, Ayanian J, Binkley N, Carr DS, Coe C, & Williams D (2019). Midlife in the United States (MIDUS 3). 2013-2014 Inter-University Consortium for Political and Social Research [distributor]. 10.3886/ICPSR36346.v7 [DOI] [Google Scholar]
- Ryff C, Almeida DM, Ayanian J, Carr DS, Cleary PD, Coe C, & Williams D (2017). Midlife in the United States (MIDUS 2). 2004-2006 Inter-University Consortium for Political and Social Research [distributor]. 10.3886/ICPSR04652.v7 [DOI] [Google Scholar]
- Savalei V, & Rhemtulla M (2012). On obtaining estimates of the fraction of missing information from full information maximum likelihood. Structural Equation Modeling, 19(3), 477–494. 10.1080/10705511.2012.687669 [DOI] [Google Scholar]
- Shim E-J, Noh H-L, Yoon J, Mun HS, & Hahm B-J (2019). A longitudinal analysis of the relationships among daytime dysfunction, fatigue, and depression in college students. Journal of American College Health, 67(1), 51–58. 10.1080/07448481.2018.1462819 [DOI] [PubMed] [Google Scholar]
- Sivertsen B, Salo P, Mykletun A, Hysing M, Pallesen S, Krokstad S, & Øverland S (2012). The bidirectional association between depression and insomnia: The HUNT study. Psychosomatic Medicine, 74(7), 758–765. 10.1097/PSY.0b013e3182648619 [DOI] [PubMed] [Google Scholar]
- Stahl ST, Smagula SF, Rodakowski J, Dew MA, Karp JF, Albert SM, & Reynolds CF (2020). Subjective Sleep Quality and Trajectories of Interleukin-6 in Older Adults. The American Journal of Geriatric Psychiatry. 10.1016/j.jagp.2020.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel Z, Marnane C, Iranpour C, Chey T, Jackson JW, Patel V, & Silove D (2014). The global prevalence of common mental disorders: a systematic review and meta-analysis 1980–2013. International Journal of Epidemiology, 43(2), 476–493. 10.1093/ije/dyu038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Shi L, Bao Y, Sun Y, Shi J, & Lu L (2018). The bidirectional relationship between sleep duration and depression in community-dwelling middle-aged and elderly individuals: evidence from a longitudinal study. Sleep Medicine, 52, 221–229. 10.1016/j.sleep.2018.03.011 [DOI] [PubMed] [Google Scholar]
- Swendsen JD (1997). Anxiety, depression, and their comorbidity: An experience sampling test of the helplessness-hopelessness theory. Cognitive Therapy and Research, 21(1), 97–114. 10.1023/A:1021872410824 [DOI] [Google Scholar]
- Tan X, Alén M, Wang K, Tenhunen J, Wiklund P, Partinen M, & Cheng S (2016). Effect of six-month diet intervention on sleep among overweight and obese men with chronic insomnia symptoms: A randomized controlled trial. Nutrients, 8(11), 751. 10.3390/nu8110751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhmann S, Beesdo-Baum K, Becker ES, & Hoyer J (2010). Specificity of interpersonal problems in generalized anxiety disorder versus other anxiety disorders and depression. Journal of Nervous and Mental Disease, 198(11), 846–851. 10.1097/NMD.0b013e3181f98063 [DOI] [PubMed] [Google Scholar]
- van der Zwan JE, de Vente W, Tolvanen M, Karlsson H, Buil JM, Koot HM, & Karlsson L (2017). Longitudinal associations between sleep and anxiety during pregnancy, and the moderating effect of resilience, using parallel process latent growth curve models. Sleep Medicine, 40, 63–68. 10.1016/j.sleep.2017.08.023 [DOI] [PubMed] [Google Scholar]
- Waller KL, Mortensen EL, Avlund K, Osler M, Fagerlund B, Lauritzen M, & Jennum P (2016). Subjective sleep quality and daytime sleepiness in late midlife and their association with age-related changes in cognition. Sleep Medicine, 17, 165–173. 10.1016/j.sleep.2015.01.004 [DOI] [PubMed] [Google Scholar]
- Wang CF, Sun YL, & Zang HX (2014). Music therapy improves sleep quality in acute and chronic sleep disorders: A meta-analysis of 10 randomized studies. International Journal of Nursing Studies, 51(1), 51–62. 10.1016/j.ijnurstu.2013.03.008 [DOI] [PubMed] [Google Scholar]
- Wittchen H-U (1994). Reliability and validity studies of the WHO-Composite International Diagnostic Interview (CIDI): A critical review. Journal of Psychiatric Research, 28(1), 57–84. 10.1016/0022-3956(94)90036-1 [DOI] [PubMed] [Google Scholar]
- Xie Y, Liu S, Chen XJ, Yu HH, Yang Y, & Wang W (2021). Effects of exercise on sleep quality and insomnia in adults: A systematic review and meta-analysis of randomized controlled trials. Frontiers in Psychiatry, 12, Article 664499. 10.3389/fpsyt.2021.664499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Li M, Pu L, Wang S, Wu J, Ruan L, & Jiang W (2017). Sleep was associated with depression and anxiety status during pregnancy: A prospective longitudinal study. Archives of Women’s Mental Health, 20(5), 695–701. 10.1007/s00737-017-0754-5 [DOI] [PubMed] [Google Scholar]
- Yuan KH, Hayashi K, & Yanagihara H (2007). A class of population covariance matrices in the bootstrap approach to covariance structure analysis. Multivariate Behavioral Research, 42(2), 261–281. 10.1080/00273170701360662 [DOI] [PubMed] [Google Scholar]
- Zainal NH, & Newman MG (2018). Executive function and other cognitive deficits are distal risk factors of generalized anxiety disorder 9 years later. Psychological Medicine, 48(12), 2045–2053. 10.1017/S0033291717003579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainal NH, & Newman MG (2021aaa). Depression and executive functioning bidirectionally impair one another across 9 years: Evidence from within-person latent change and cross-lagged models. European Psychiatry, 64(1), Article e43. 10.1192/j.eurpsy.2021.2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainal NH, & Newman MG (2021bbb). Larger increase in trait negative affect is associated with greater future cognitive decline and vice versa across 23 years. Depression and Anxiety, 38(2), 146–160. 10.1002/da.23093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainal NH, & Newman MG (2021c). Within-person increase in pathological worry predicts future depletion of unique executive functioning domains. Psychological Medicine, 51(10), 1676–1686. 10.1017/S0033291720000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainal NH, & Newman MG (2022). Inflammation mediates depression and generalized anxiety symptoms predicting executive function impairment after 18 years. Journal of Affective Disorders, 296, 465–475. 10.1016/j.jad.2021.08.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainal NH, & Newman MG (in press). Depression and worry symptoms predict future executive functioning impairment via inflammation. Psychological Medicine. 10.1017/S0033291721000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LN, Zong QQ, Yang Y, Zhang L, Xiang YF, Ng CH, & Xiang YT (2020). Gender difference in the prevalence of insomnia: A meta-analysis of observational studies. Frontiers in Psychiatry, 11, Article 577429. 10.3389/fpsyt.2020.577429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.-j., Yan C, Shum D, & Deng C.-p. (2020). Responses to academic stress mediate the association between sleep difficulties and depressive/anxiety symptoms in Chinese adolescents. Journal of Affective Disorders, 263, 89–98. 10.1016/j.jad.2019.11.157 [DOI] [PubMed] [Google Scholar]
- Zhao M, Tuo H, Wang S, & Zhao L (2020). The effects of dietary nutrition on sleep and sleep disorders. Mediators of Inflammation, 2020, Article 3142874. 10.1155/2020/3142874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D.-m., Zhang C, Yang Y, Zhang Y, Zhao W, Zhang B, & Yu Y (2020). The relationship between sleep efficiency and clinical symptoms is mediated by brain function in major depressive disorder. Journal of Affective Disorders, 266, 327–337. 10.1016/j.jad.2020.01.155 [DOI] [PubMed] [Google Scholar]
- Zou P, Wang X, Sun L, Liu K, Hou G, Yang W, & Chen Q (2020). Poorer sleep quality correlated with mental health problems in college students: A longitudinal observational study among 686 males. Journal of Psychosomatic Research, 136, Article 110177. 10.1016/j.jpsychores.2020.110177 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We have shared the link to our code and output at the Attach File step.


