Abstract
Numerous studies have reported that tangeretin is a polymethoxylated flavone with a variety of biological activates, but little research has been done on the antioxidant mechanism of tangeretin. Hence, we investigated the effect of tangeretin on the nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant response element (ARE) pathway and its potential molecular mechanisms by in vitro and in silico research. The results of molecular docking suggested that tangeretin bound at the top of the central pore of Kelch-like ECH-associated protein 1 (Keap1) Kelch domain, and the hydrophobic and hydrogen bond interactions contributed to their stable binding. Herein, the regulation of Nrf2-ARE pathway by tangeretin was explored in the human embryonic kidney cell line HEK293T, which is relatively easy to be transfected. Upon binding to tangeretin, Nrf2 translocated to the nucleus of HEK293T cells, which in turn activated the Nrf2-ARE pathway. Luciferase reporter gene analysis showed that tangeretin significantly induced ARE-mediated transcriptional activation. Real-time PCR and Western blot assays showed that tangeretin induced the gene and protein expressions of Nrf2-mediated targets, including heme oxygenase 1 (HO-1), nicotinamide adenine dinucleotide phosphate (NADPH) quinone dehydrogenase 1 (NQO1), and glutamate-cysteine ligase (GCLM). In addition, tangeretin could effectively scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals. In summary, tangeretin may be a potential antioxidant via activating the Nrf2-ARE pathway.
Keywords: Tangeretin, Nuclear factor erythroid 2-related factor 2, Antioxidant response element, Molecular docking
Graphical abstract

Highlights
-
•
Tangeretin bound to Kelch-like ECH-associated protein 1 (Keap1) Kelch domain.
-
•
Tangeretin enhanced nuclear factor erythroid 2-related factor 2 (Nrf2) translocation.
-
•
Tangeretin induced Nrf2-mediated gene and protein expressions.
-
•
Tangeretin induced antioxidant response element (ARE)-mediated transactivation.
-
•
Tangeretin exhibited antioxidant effect via activating Keap1-Nrf2-ARE signaling.
1. Introduction
Flavonoids are a class of polyphenolic compounds with 2-phenyl flavone structure, and are generally referred to as a general term for a class of compounds with a C6-C3-C6 structure consisting of two benzene rings interconnected by three carbon atoms (Kato et al., 2023; Li et al., 2022a; Lu et al., 2022; Müller et al., 2022; Tan et al., 2022). They are widely present in citrus, grapes, tea, chamomile, cocoa (Schijlen et al., 2004). Reportedly, more than 10 thousands flavonoids have been separated and identified from plants (Agati et al., 2012). Recent studies indicate that flavonoids have multiple benefits for human health and are therefore widely used in health foods, pharmaceuticals and cosmetics (Liang et al., 2021; Zhang et al., 2021; Zou et al., 2021).
Tangeretin (5,6,7,8,4′-pentamethoxyflavone), a member of polymethoxylated flavone, is a natural active substance containing two aromatic rings, a heterocyclic pyran ring, and a total of five methoxy groups (Raza et al., 2020). It is present in the peel of citrus fruits such as sweet orange (Citrus sinensis), as well as in the dried tangerine peel, a traditional Chinese medicine (Citri reticulatae pericarpium) (Ho and Kuo, 2014). Tangeretin has been reported to have a variety of biological activities, showing in particular antioxidant, antidiabetic, anti-inflammatory and neuroprotective effects (Guo et al., 2017; Lakshmi and Subramanian, 2014; Ma et al., 2016; Wang et al., 2018). In addition, it also showed good efficacy in the treatment of breast cancer, bladder cancer, ovarian cancer and gastric cancer (Chen et al., 2014; Ho and Kuo, 2014).
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a member of the basic leucine zipper transcription family that regulates the expression of several antioxidant proteins and phase II detoxification enzymes (Ji et al., 2015). Under normal cellular conditions, Nrf2 is anchored to cytoplasmic actin by interacting with the inhibitory protein Kelch-like ECH-associated protein 1 (Keap1) and directs rapid proteasomal degradation and ubiquitination (Peng et al., 2015; Ren et al., 2021; Zhang et al., 2020b). Interestingly, natural active substance is able to bind competitively to the Keap1 protein, prompting the dissociation of Nrf2 from Keap1. Nrf2 is released from Keap1 translocates into the nucleus, where it forms a dimer with transcription factors and binds to the antioxidant response element (ARE) in the promoter regions of the target genes (Ai et al., 2022; Ding et al., 2022; Han et al., 2022; Li et al., 2022b; Ma et al., 2023). This in turn activates the Nrf2-ARE signaling pathway and increases Nrf2-mediated expression of downstream antioxidant genes and proteins, including heme oxygenase 1 (HO-1), nicotinamide adenine dinucleotide phosphate (NADPH) quinone dehydrogenase 1 (NQO1), and glutamate-cysteine ligase (GCLM) (Kensler et al., 2007; Li et al., 2018; Liu et al., 2017).
The Nrf2-ARE is the most critical antioxidant signaling pathway in biological organisms (Dai et al., 2022; Guo et al., 2022; Jiang et al., 2022; Qiu et al., 2022; Shen et al., 2022a; Yan et al., 2022). So far, accumulating evidence has shown that a large amount of natural active substances activate Nrf2-mediated antioxidant signaling pathway and regulate the expression of cytoprotective enzymes, thus playing an important role in preventing antioxidant damage (Eggler et al., 2010). Several natural antioxidants have been screened and selected by the Nrf2-ARE pathway, including resveratrol (Agrawal et al., 2013), curcumin (Chen et al., 2020), epicatechin (Bahia et al., 2008) and quercetin (Arredondo et al., 2010). However, research on the effect of tangeretin on the activation of Nrf2-ARE signaling pathway is still limited and its molecular mechanism has not been fully elucidated.
This work aims to identify tangeretin (Fig. 1) as a potential antioxidant targeting the Keap1-Nrf2-ARE signaling pathway. In order to investigate whether tangeretin can target Keap1, molecular docking was performed to explore their binding interaction. Herein, the regulation of Nrf2-ARE pathway by tangeretin was explored in the human embryonic kidney cell line HEK293T, which is relatively easy to be transfected. The cytotoxicity of tangeretin on HEK293T cells was subsequently evaluated. The effect of tangeretin on Nrf2 nuclear translocation was investigated by measuring Nrf2 protein expression levels. The effect of tangeretin on ARE-luciferase activity was evaluated by the luciferase reporter gene assay. The expression of three Nrf2-mediated target genes was evaluated by mRNA analysis. The protein expression levels of 3 antioxidant protease were detected by Western blot. In addition, its ability to scavenge free radical was examined by the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay.
Fig. 1.
Structures of tangeretin (5,6,7,8,4′-pentamethoxyflavone).
2. Materials and methods
2.1. Materials
Dulbecco's modified Eagle medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, NY, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), bovine serum albumin (BSA), and dimethyl sulfoxide (DMSO) were obtained from Fluka (Sigma-Aldrich, Shanghai, China). Tangeretin and tert-butylhydroquinone were purchased from Yuanye Biotechnology Co., Ltd. (Shanghai, China). DPPH free radical scavenging capacity assay kit was purchased from Nanjing Jiancheng Technology Co., Ltd. (Nanjing, China). Lipofectamine 2000 transfection reagent was purchased from Thermo Fisher Science (San Jose, CA, USA). Trizol reagent, reverse transcriptional kit and quantitative polymerase chain reaction (PCR) kit were purchased from Transgen Biotech Ltd. (Beijing, China). Anti-glyceraldhyde-3-phosphate dehydrogenase (GAPDH) and anti-Lamin B1 were purchased from Sino Biological (Beijing, China). Anti-HO-1, anti-NQO1, and anti-GCLM were obtained from Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China).
2.2. Molecular docking between tangeretin and Keap1
In order to investigate whether tangeretin can target Keap1, molecular docking was performed to explore their binding interaction. The crystal structure of the Kelch domain of human Keap1 (PDB 4L7B) in complex with ligand (S,R,S)-1a, namely (1S,2R)-2-[(1S)-1-[(1,3-dioxo-2,3-dihydro-1H-isoindol-2-yl)methyl]-1,2,3,4-tetrahydroisoquinolin-2-carbonyl]cyclohexane-1-carboxylic acid, was obtained from Protein Data Bank (Jnoff et al., 2014). This crystal structure of Homo sapiens keap1 kelch domain was extracted by X-ray diffraction method, with a resolution of 2.41 Å. The co-crystallized ligand was removed by Chimera and the preparation of Keap1 Kelch domain was performed by AutoDockTools-1.5.6. The 3D structure of tangeretin was developed and optimized by GaussView 5.0 and Gaussian 09W, respectively. After verification of simulation protocol by re-docking the co-crystallized ligand with Keap1 Kelch domain, tangeretin was docked into the ligand binding site. A grid box of 40 × 40 × 40 points with a grid space of 0.375 Å was set at the stereocenter of the co-crystallized ligand. The Lamarckian genetic algorithm (LGA) was chosen to dock conformationally flexible ligand tangeretin with Keap1 Kelch domain in AutoDockTools-1.5.6. The docking parameters were set by default, namely population size is 150, number of GA runs is 10, maximum numbers of generations and evals are 27000 and medium, rates of gene mutation and crossover are 0.02 and 0.8. The detailed interaction between tangeretin and Keap1 was visualized by PyMOL.
2.3. Cell culture and MTT assay
Human embryonic kidney cell line HEK293T, obtained from the Cell Bank of the Chinese Academy of Science (Shanghai, China), was cultured in DMEM supplemented with 10% FBS at 37°C with 5% CO2.
HEK293T cell (1 × 104 cells/well) were seeded in 96-well plates and cultured for 18 h. Cells were treated with different concentrations of tangeretin or H2O2 for 24 h. Added 10% MTT for 4 h. The MTT-containing medium was discarded and 150 μL DMSO was added. The absorbance was measured at 570 nm with a microlate reader SpectraMax i3x (Molecular Devices, Sunnyvale, CA, USA).
2.4. Construction of the plasmids
The pARE-Luc (reporter plasmid) was synthesized by ligating a double-stranded oligonucleotide tandem ARE target fragment containing the Nrf2 binding site spanning 5′-TGACTCAGC-3′ into a luciferase reporter gene plasmid vector (Tsai et al., 2011; Yang et al., 2013). Plasmid pRL-SV40 (Promega, WI, USA) was used as the internal control.
2.5. Luciferase reporter gene assay
HEK293T cells (1 × 105 cells/well) were seeded in 24-well plates and incubated for 18 h. The cells were transiently transfected with plasmids containing pARE-Luc and pRL-SV40 using Lipofectamine 2000 according to manufacturer's instruction. After 4 h of transfection, the test compounds tert-butylhydroquinone or tangeretin were added and incubated for 24 h followed by the cell lysis using passive lysis buffer. The luciferase activities were measured on a microplate reader SpectraMax i3x using Dual-Glo Luciferase Assay System (Promega, Madison, WI, USA).
2.6. Real-time quantitative polymerase chain reaction analysis
HEK293T cells (1 × 105 cells/well) were incubated in 6-well plates for 18 h in 2 mL DMEM medium, and treated with different concentrations of tangeretin for 18 h, respectively, followed by exposure to 500 μM of H2O2 for 8 h. Total RNA was extracted with the Trizol reagent according to the manufacturer's protocol. The reverse transcriptional kit was used to perform reverse transcription reactions with 1000 ng of RNA per sample. Real-time quantitative PCR analysis was conducted using quantitative PCR kit. The general PCR parameters were 94°C for 10 min; 40 circles of 94°C for 5 s, 60°C for 30s; 94°C for 10 s, 65°C for 60 s, 97°C for 10s; cooling to 37°C for 30s. Primers used in this study were shown in Table 1. Relative expression of heme oxygenase-1 (HO-1), nicotinamide adenine dinucleotide phosphate (NADPH) quinone dehydrogenase 1 (NQO1) and glutamate-cysteine ligase (GCLM) was calculated by the ΔΔCt method with normalization to GAPDH (Zhang et al., 2020a).
Table 1.
Primer sequences for quantitative real-time PCR.
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) | NCBI ID |
|---|---|---|---|
| HO-1 | GGCAGAGGGTGATAGAAGAGG | TAAGGACCCATCGGAGAAGC | NM_002133.3 |
| NQO1 | GCCGAGTCTGTTCTGGCTTATT | CATGGCAGCGTAAGTGTAAGCA | NM_001286137.2 |
| GCLM | AACTGACTTAGGAGCATAACTTACC | TATCTGCCTCAATGACACCA | NM_002061.4 |
| GAPDH | GAAGACGGGCGGAGAAAC | GCCCAATACGACCAAATCCG | NM_002046.7 |
2.7. Western blot analysis
HEK293T cells were seeded in 10 cm discs at a density of 5 × 106 cells for 24 h. Total protein, cytoplasmic protein and nuclear protein were extracted, and the protein concentration was determined using BCA protein detection kit. Protein samples were resolved on SDS-PAGE gel electrophoresis and transferred onto PVDF membranes. PVDF membranes were blocked with 3% BSA for 2 h at room temperature and incubated with primary antibodies overnight at 4°C. Anti-HO-1, anti-NQO1, anti-GCLM, anti-GAPDH and anti-Lamin B1 were used as primary antibodies. Subsequently, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies. The protein bands were detected using an enhanced chemiluminescence (ECL) immunoblotting detection kit.
2.8. Free radical-scavenging assay
The free radical scavenging ability of tangeretin was determined according to the protocol of the DPPH kit manufacturer, after the modification of the previous method (Mutlu-Ingok et al., 2021; Yao et al., 2014). Briefly, 80 μL tangeretin solution was added to 120 μL DPPH anhydrous ethanol solution and the mixture was allowed to stand in the dark at room temperature for 30 min. The absorbance value was measured at 517 nm. The DPPH radical scavenging rate was calculated using the following equation:
| DPPH radical scavenging rate (%) = (Ac - As)/Ac × 100% |
As represents the absorbance of the measured sample and Ac represents the absorbance of the blank.
2.9. Statistical analysis
All assays were performed independently in triplicate and data were presented as mean ± standard deviation (SD). Statistical analysis was performed using one-way SPSS program (SPSS Inc., Chicago, IL, USA). Tukey's multiple comparison test was conducted at a 95% confidence level. The results were considered statistically significant as *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the control.
3. Results and discussion
3.1. Binding interaction between tangeretin and Keap1
In this work, the ligand (S,R,S)-1a was re-docked with Keap1 Kelch domain firstly. As shown in Fig. 2, the re-docked ligand orientation was almost overlaid with the co-crystallized ligand orientation, indicating that the simulation protocol reliably reproduced the binding mode between (S,R,S)-1a and Keap1 Kelch domain. Then tangeretin was docked with Keap1 Kelch domain and the result can be seen in Fig. 3. Obviously, tangeretin bound at the top of the central pore of Keap1 Kelch domain, and assumed an orientation that was similar to the co-crystallized ligand. In addition to the hydrophobic interactions, tangeretin formed a hydrogen bond with Ser602 and two hydrogen bonds with Asn414. To sum up, the hydrophobic and hydrogen bond interactions contributed to the stable binding between tangeretin and Keap1. The binding free energy of tangeretin with Keap1 was −6.21 kcal/mol, indicating that tangeretin was a ligand of Keap1. Previous in vivo studies have shown that tangeretin can attenuate oxidative stress in the liver of 7,12-dimethylbenz[a]anthracene induced rats and myocardial oxidative injury in fatigued mice through targeting Keap1 (Arivazhagan and Subramanian, 2015; Kou et al., 2019), which are consistent with the in silico findings herein.
Fig. 2.
Computational alignment of re-docked ligand (S,R,S)-1a (cyan sticks) and co-crystallized ligand (S,R,S)-1a (magenta sticks) at the top of the central pore of Keap1 Kelch domain. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
The result of molecular docking between tangeretin (green sticks) and Keap1 Kelch domain. Co-crystallized ligand (S,R,S)-1a, magenta sticks; hydrogen bonds, red dotted lines. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. Cytotoxicity of tangeretin or H2O2 in HEK293T cells
The cytotoxicity of tangeretin or H2O2 in HEK293T cells was evaluated by MTT assay (Fig. 4). The high concentration of tangeretin significantly inhibited the growth of HEK293T cells compared with the control group. Notably, tangeretin significantly inhibited the growth of HEK293T cells in a dose-dependent manner. The IC50 values of tangeretin was 500 μM (p < 0.001). Fig. 4A showed that tangeretin at a concentration of 50 μM exhibited cytotoxic effect against HEK293T cells. Therefore, 10, 20 and 40 μM were chosen for subsequent experiments.
Fig. 4.
Cytotoxicities of tangeretin (TAN) (A) and H2O2 (B). Results are given as means ± SD of three independent experiments and normalized to DMSO control group. *, **, ***, statistically significant differences (p < 0.05, p < 0.01, p < 0.001).
H2O2 is a reactive oxygen species that induces oxidative cellular damage (Jin et al., 2016). Exposure to 400 or 500 μM of H2O2 significantly decreased the viability of HEK293T cells up to 65.45 ± 1.02% and 50.93 ± 3.38%, respectively (Fig. 4B). The IC50 value for H2O2 was 500 μM (p < 0.001). Therefore, 500 μM of H2O2 was chosen to induce oxidative damage in HEK293T cells.
3.3. Tangeretin induced the nuclear translocation of Nrf2 in HEK293T cells
To determine the effect of tangeretin on Nrf2 nuclear translocation, the Nrf2 protein expression levels in the cytoplasm and nucleus of HEK293T cells after treatment with different concentrations of tangeretin were examined by Western blot. HEK293T cells were co-incubated with different concentrations of tangeretin for 18 h and then exposed to 500 μM H2O2 for 8 h. The nuclear protein expression level of Nrf2 was up-regulated by positive control tert-butylhydroquinone treatment (Fig. 5). Compared with the H2O2 group, different concentrations of tangeretin significantly increased Nrf2 nuclear protein expression in HEK293T cells in a dose-dependent manner and reached a maximum at 40 μM. As shown in Fig. 5D, Nrf2 nuclear protein/Nrf2 cytoplasmic protein was increased, indicating that tangeretin was able to promote Nrf2 nuclear translocation. Under normal conditions, Nrf2 binds to Keap1, which anchors Nrf2 in the cytoplasm and allowing its continuous degradation via the proteasome during ubiquitin-mediated processed (Hong et al., 2022; Puppala et al., 2022; Shen et al., 2022b). Upon binding to tangeretin, Nrf2 dissociates from Keap1 and Nrf2 translocated to the nucleus. Upon the activation by tangeretin, Nrf2 can bind to ARE, which in turn promotes Nrf2-mediated expression of target genes and proteins. The binding of Nrf2 and ARE in the nucleus is an essential mechanism for inducing the expression of antioxidant genes and proteins.
Fig. 5.
Effect of tangeretin on nuclear translocation of Nrf2. (A) The expression levels of nuclear Nrf2 and cytosolic Nrf2 in HEK293T cells were detected by Western blot after treatment with tangeretin for 18 h, followed by 500 μM H2O2 for 8 h. (B) The levels of nuclear Nrf2 were quantified by densitometry. (C) The levels of cytosolic Nrf2 were quantified by densitometry. (D) The levels of nuclear Nrf2/cytosolic Nrf2 were quantified by densitometry. Each group of experiments was determined three times and given as mean ± SD. **, ***, statistically significant differences (p < 0.01 and p < 0.001).
3.4. Tangeretin activated the transcriptional activity of ARE in HEK293T cells
The luciferase reporter gene is an effective method for screening antioxidant drugs to activate the Nrf2-ARE pathway (Liang et al., 2019), and therefore can be used to assess the potential role of tangeretin on ARE-driven luciferase activity. tert-butylhydroquinone is a typically strong antioxidant inducer and can be used as a positive control to evaluate the antioxidant activity of tangeretin (Hara et al., 2003). It can be observed that the luciferase activity of HEK293T cells was significantly induced up to 12.65 ± 0.89-fold in the presence of 12.5 μM tert-butylhydroquinone. As shown in Fig. 6, tangeretin at concentrations of 10, 20 and 40 μM significantly induced trans-activation of ARE by 1.69 ± 0.15-fold, 3.56 ± 0.16-fold and 5.13 ± 0.25-fold, respectively, compared to the control (p < 0.01). Based on this result, tangeretin induced ARE transactivation in a dose-responsive manner, confirming that tangeretin is a potential ARE activator. Other natural active substances have similar effects, such as genistein, daidzein, formononetin and biochanin A (BCA) which also exhibit ARE transactivation (Liang et al., 2019).
Fig. 6.
HEK293T cells were treated with tangeretin or tert-butylhydroquinone (t-BHQ) for 24 h, and then the luciferase activity was measured. Results are given as means ± SD of three independent experiments and normalized to DMSO control group. **, ***, statistically significant differences (p < 0.01 and p < 0.001).
3.5. Tangeretin induced the gene expression of HO-1, NQO1, GCLM in HEK293T cells
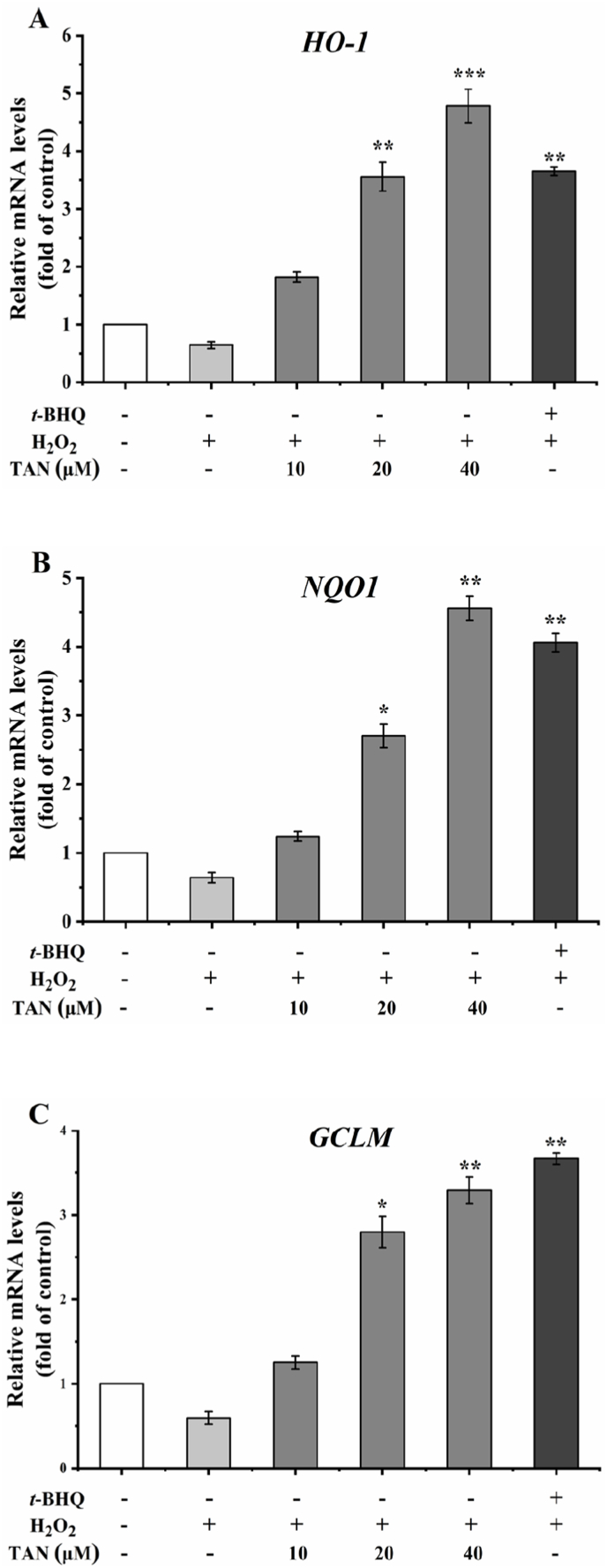
To verify the potential antioxidant mechanism of tangeretin, we further examined the expression levels of tangeretin on three Nrf2-mediated target genes, including HO-1, NQO1 and GCLM. The real-time PCR results showed that the mRNA levels of all three Nrf2-mediated target genes were up-regulated after treatment with tert-butylhydroquinone at a concentration of 12.5 μM in the presence of H2O2. In contrast, H2O2 down-regulated the mRNA expression levels of the three genes compared to the control group. We then investigated the effects of tangeretin at concentrations of 10, 20 and 40 μM on the mRNA expression levels of three Nrf2-mediated target genes. As shown in Fig. 7, tangeretin significantly promoted the mRNA expression of HO-1, NQO1 and GCLM in a dose-dependent manner after treatment with tangeretin in the presence of H2O2 (p < 0.05).
Fig. 7.
Effect of tangeretin on the expression of Nrf2-mediated target gene. Tangeretin was used for 18 h, followed by 500 μM H2O2 for 8 h. (A) (B) and (C) are the relative mRNA levels of HO-1, NQO1 and GCLM in HEK293T cells, respectively. Results are given as means ± SD of three independent experiments and normalized to H2O2 control group. *, **, ***, statistically significant differences (p < 0.05, p < 0.01 and p < 0.001, respectively).
Keap1-Nrf2-ARE is the most important antioxidant signaling pathway in human body. Nrf2 is an important transcription factor that regulates the antioxidant response, and Nrf2 binding to ARE induces the expression of cytoprotease genes including HO-1, NQO1 and GCLM, hence playing an important role in maintaining cellular redox homeostasis. It has been demonstrated that in the presence of certain natural active substances, Nrf2 nuclear translocation binds to the ARE, which in turn regulates Nrf2-mediated target gene expression (Itoh et al., 1999; Lu et al., 2016; Wild et al., 1999). The results of the present study are consistent with previous studies in which tangeretin increased the mRNA expression levels of three Nrf2-mediated target genes, including HO-1, NQO1 and GCLM (Liang et al., 2018).
3.6. Tangeretin induced the protein expression of HO-1, NQO1 and GCLM in HEK293T cells
To determine the effect of tangeretin on proteins expression level, HEK293T cells were co-incubated with tangeretin for 18 h and then exposed to 500 μM H2O2 for 8 h. Subsequently, we detected the proteins expression level of HO-1, NQO1 and GCLM by Western blot (Fig. 8A). H2O2 effectively reduced the expression of three proteins compared to the control. In addition, we observed that the expression of HO-1, NQO1 and GCLM proteins significantly increased in HEK293T cells after tangeretin treatment compared with the H2O2 group (Fig. 8B, C and D). Consistent with the results of mRNA, tangeretin induced the expression of HO-1, NQO1 and GCLM proteins. It was further confirmed that tangeretin could effectively activate the Nrf2-ARE antioxidant signaling pathway. It showed that tangeretin activation of mitogen-activated protein kinase (MAPK)-Nrf2-ARE signaling pathway can protect HepG2 cells from tert-butyl hydroperoxide-induced oxidative damage (Liang et al., 2018). This is consistent with the present study and suggests that tangeretin is a potential antioxidant.
Fig. 8.
Effect of tangeretin on the protein levels of HO-1, NQO1 and GCLM. (A) The expression levels of HO-1, NQO1 and GCLM in HEK293T cells were detected by Western blot after treatment with tangeretin for 18 h, followed by 500 μM H2O2 for 8 h. (B) The levels of HO-1 were quantified by densitometry. (C) The levels of NQO1 were quantified by densitometry. (D) The levels of GCLM were quantified by densitometry. Each group of experiments was determined three times and given as mean ± SD. *, ***, statistically significant differences (p < 0.05 and p < 0.001).
3.7. Scavenging of free radicals
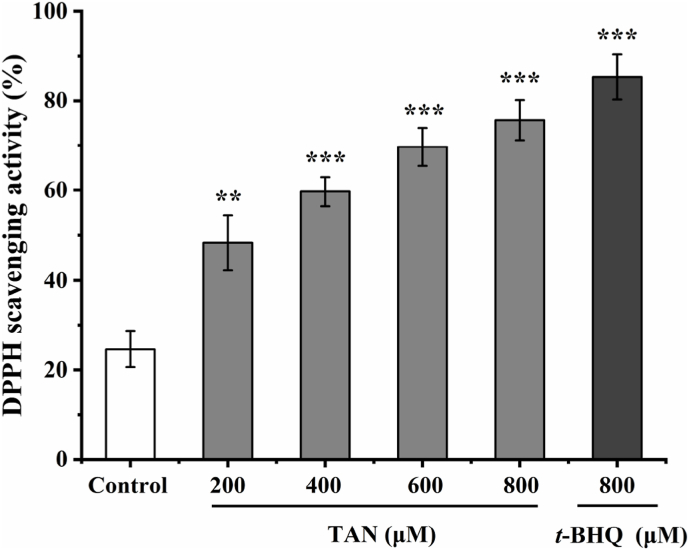
The DPPH radical scavenging assay can be used to screen for antioxidants (Wang et al., 2021). To investigate the free radical scavenging ability of different concentrations of tangeretin, we measured it by DPPH assay. Fig. 9 showed that both tert-butylhydroquinone and tangerine have the ability to scavenge DPPH free radicals. The scavenging of DPPH radicals by tert-butylhydroquinone at a concentration of 800 μM was significant. Tangeretin scavenges DPPH radicals in a dose-dependent manner, increasing to 75.67 ± 4.51% at 800 μM. Similarly, 6-shogaol, 6-dehydroginger and 6-gingerol, tangeretin contains phenoxy group, which is effective in scavenging free radicals and plays a key role in antioxidant activity (Peng et al., 2015). DPPH assay data further confirmed the antioxidant ability of tangeretin.
Fig. 9.
Scavenging effect of tangeretin and tert-butylhydroquinone on DPPH free radicals. Results are given as means ± SD of three independent experiments and normalized to DMSO control group. **, ***, statistically significant differences (p < 0.01 and p < 0.001).
4. Conclusions
This work investigated the regulation of tangeretin on the Keap1-Nrf2-ARE signaling pathway by a combination of in vitro and in silico approaches. The results of molecular docking suggested that tangeretin bound at the top of the central pore of Keap1 Kelch domain, and the hydrophobic and hydrogen bond interactions contributed to their stable binding. Western blot results showed that tangeretin can translocate Nrf2 from the cytoplasm into the nucleus of HEK293T cells. Tangeretin significantly increased ARE luciferase activity. With respect to the Nrf2-mediated targets, tangeretin not only increased the mRNA expression of HO-1, NQO1 and GCLM, but also up-regulated their protein expression levels. Moreover, tangeretin effectively scavenged DPPH free radicals in a dose-dependent manner. In summary, tangeretin may be a potential antioxidant targeting the Nrf2-ARE signaling pathway.
CRediT authorship contribution statement
Chengyu Lv: Investigation, Writing – original draft. Yuqiu Li: Investigation, Writing – original draft. Rong Liang: Investigation, Methodology. Wei Huang: Investigation, Methodology. Yechen Xiao: Investigation, Methodology. Xinqi Ma: Investigation, Methodology. Yongjun Wang: Investigation, Methodology. Haoyang Zou: Investigation, Data curation. Fen Qin: Investigation, Data curation. Chang Sun: Investigation, Data curation. Tiezhu Li: Project administration, Writing – review & editing. Jie Zhang: Project administration, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Key R&D Program Intergovernmental International Science and Technology Innovation Cooperation Project (2022YFE0130300), the Natural Science Foundation of Shandong Province (ZR2020QC220), the Science and Technology Development Project Foundation of Jilin Province (20210203182SF), the Capital Construction Funds within the Provincial Budget in 2022 (2022c043-12), and the Agricultural Science and Technology Innovation Program of Jilin Province (CXGC2021TD006).
Handling Editor: A.G. Marangoni
Contributor Information
Tiezhu Li, Email: tiezhu_li@163.com.
Jie Zhang, Email: zhangjie83@jlu.edu.cn.
References
- Agati G., Azzarello E., Pollastri S., Tattini M. Flavonoids as antioxidants in plants: location and functional significance. Plant Sci. 2012;196:67–76. doi: 10.1016/j.plantsci.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Agrawal M., Kumar V., Singh A.K., Kashyap M.P., Khanna V.K., Siddiqui M.A., Pant A.B. trans-Resveratrol protects ischemic PC12 cells by inhibiting the hypoxia associated transcription factors and increasing the levels of antioxidant defense enzymes. ACS Chem. Neurosci. 2013;4(2):285–294. doi: 10.1021/cn300143m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai G., Wu X., Dou Y., Huang R., Zhong L., Liu Y., Xian Y., Lin Z., Li Y., Su Z., Chen J., Qu C. Oxyberberine, a novel HO-1 agonist, effectively ameliorates oxidative stress and inflammatory response in LPS/D-GalN induced acute liver injury mice via coactivating erythrocyte metabolism and Nrf2 signaling pathway. Food Chem. Toxicol. 2022;166 doi: 10.1016/j.fct.2022.113215. [DOI] [PubMed] [Google Scholar]
- Arivazhagan L., Subramanian S.P. Tangeretin, a citrus flavonoid attenuates oxidative stress and protects hepatocellular architecture in rats with 7, 12- dimethylbenz(a)anthracene induced experimental mammary carcinoma. J. Funct.Foods. 2015;15:339–353. doi: 10.1016/j.jff.2015.03.041. [DOI] [Google Scholar]
- Arredondo F., Echeverry C., Abin-Carriquiry J.A., Blasina F., Antúnez K., Jones D.P., Go Y.M., Liang Y.L., Dajas F. After cellular internalization, quercetin causes Nrf2 nuclear translocation, increases glutathione levels, and prevents neuronal death against an oxidative insult. Free Radical Biol. Med. 2010;49(5):738–747. doi: 10.1016/j.freeradbiomed.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Bahia P.K., Rattray M., Williams R.J. Dietary flavonoid (-)epicatechin stimulates phosphatidylinositol 3-kinase-dependent anti-oxidant response element activity and up-regulates glutathione in cortical astrocytes. J. Neurochem. 2008;106(5):2194–2204. doi: 10.1111/j.1471-4159.2008.05542.x. [DOI] [PubMed] [Google Scholar]
- Chen J., Zheng J., Mcclements D.J., Xiao H. Tangeretin-loaded protein nanoparticles fabricated from zein/β-lactoglobulin: preparation, characterization, and functional performance. Food Chem. 2014;158:466–472. doi: 10.1016/j.foodchem.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Chen S., Yang S., Wang M., Chen J., Li P. Curcumin inhibits zearalenone-induced apoptosis and oxidative stress in Leydig cells via modulation of the PTEN/Nrf2/Bip signaling pathway. Food Chem. Toxicol. 2020;141 doi: 10.1016/j.fct.2020.111385. [DOI] [PubMed] [Google Scholar]
- Dai X.Y., Zhu S.Y., Chen J., Li M.Z., Zhao Y., Talukder M., Li J.L. Lycopene alleviates di(2-ethylhexyl) phthalate-induced splenic injury by activating P62-Keap1-NRF2 signaling. Food Chem. Toxicol. 2022;168 doi: 10.1016/j.fct.2022.113324. [DOI] [PubMed] [Google Scholar]
- Ding X., Zhao H., Qiao C. Icariin protects podocytes from NLRP3 activation by Sesn2-induced mitophagy through the Keap1-Nrf2/HO-1 axis in diabetic nephropathy. Phytomedicine. 2022;99 doi: 10.1016/j.phymed.2022.154005. [DOI] [PubMed] [Google Scholar]
- Eggler A.L., Gay K.A., Mesecar A.D. Molecular mechanisms of natural products in chemoprevention: induction of cytoprotective enzymes by Nrf2. Mol. Nutr. Food Res. 2010;52:S84–S94. doi: 10.1002/mnfr.200700249. [DOI] [PubMed] [Google Scholar]
- Guo T., Fang X., Liu Y., Ruan Y., Hu Y., Wang X., Hu Y., Wang G., Xu Y. Acute lung inflammation induced by zinc oxide nanoparticles: evolution and intervention via NRF2 activator. Food Chem. Toxicol. 2022;162 doi: 10.1016/j.fct.2022.112898. [DOI] [PubMed] [Google Scholar]
- Guo X.Q., Cao Y.L., Hao F., Yan Z.R., Wang M.L., Liu X.W. Tangeretin alters neuronal apoptosis and ameliorates the severity of seizures in experimental epilepsy-induced rats by modulating apoptotic protein expressions, regulating matrix metalloproteinases, and activating the PI3K/Akt cell survival pathway. Adv. Med. Sci. 2017;62(2):246–253. doi: 10.1016/j.advms.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Han L., Zhang W., Wang J., Jing J., Zhang L., Liu Z., Gao A. Shikonin targets to m6A-modified oxidative damage pathway to alleviate benzene-induced testicular injury. Food Chem. Toxicol. 2022;170 doi: 10.1016/j.fct.2022.113496. [DOI] [PubMed] [Google Scholar]
- Hara H., Ohta M., Ohta K., Kuno S., Adachi T. Increase of antioxidative potential by tert-butylhydroquinone protects against cell death associated with 6-hydroxydopamine-induced oxidative stress in neuroblastoma SH-SY5Y cells. Mol. Brain Res. 2003;119(2):125–131. doi: 10.1016/j.molbrainres.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Ho S.C., Kuo C.T. Hesperidin, nobiletin, and tangeretin are collectively responsible for the anti-neuroinflammatory capacity of tangerine peel (Citri reticulatae pericarpium) Food Chem. Toxicol. 2014;71:176–182. doi: 10.1016/j.fct.2014.06.014. [DOI] [PubMed] [Google Scholar]
- Hong H., Lou S., Zheng F., Gao H., Wang N., Tian S., Huang G., Zhao H. Hydnocarpin D attenuates lipopolysaccharide-induced acute lung injury via MAPK/NF-κB and Keap1/Nrf2/HO-1 pathway. Phytomedicine. 2022;101 doi: 10.1016/j.phymed.2022.154143. [DOI] [PubMed] [Google Scholar]
- Itoh K., Ishii T., Wakabayashi N., Yamamoto M. Regulatory mechanisms of cellular response to oxidative stress. Free Radic. Res. 1999;31(4):319–324. doi: 10.1080/10715769900300881. [DOI] [PubMed] [Google Scholar]
- Ji L., Sheng Y., Zheng Z., Shi L., Wang Z. The involvement of p62-Keap1-Nrf2 antioxidative signaling pathway and JNK in the protection of natural flavonoid quercetin against hepatotoxicity. Free Radical Biol. Med. 2015;85:12–23. doi: 10.1016/j.freeradbiomed.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Jiang Q., Chen X., Tian X., Zhang J., Xue S., Jiang Y., Liu T., Wang X., Sun Q., Hong Y., Li C., Guo D., Wang Y., Wang Q. Tanshinone I inhibits doxorubicin-induced cardiotoxicity by regulating Nrf2 signaling pathway. Phytomedicine. 2022;106 doi: 10.1016/j.phymed.2022.154439. [DOI] [PubMed] [Google Scholar]
- Jin X., Wang K., Liu H., Hu F., Liu J. Protection of bovine mammary epithelial cells from hydrogen peroxide-induced oxidative cell damage by resveratrol. Oxid. Med. Cell. Longev. 2016 doi: 10.1155/2016/2572175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jnoff E., Albrecht C., Barker J.J., O B., Beaumont E., Bromidge S. Binding mode and structure-activity relationships around direct inhibitors of the Nrf2-Keap1 complex. ChemMedChem. 2014;9(4):699–705. doi: 10.1002/cmdc.201300525. [DOI] [PubMed] [Google Scholar]
- Kato K., Takahashi M., Oh-hashi K., Ando K., Hirata Y. Quercetin and resveratrol inhibit ferroptosis independently of Nrf2-ARE activation in mouse hippocampal HT22 cells. Food Chem. Toxicol. 2023;172 doi: 10.1016/j.fct.2022.113586. [DOI] [PubMed] [Google Scholar]
- Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Kou G., Wu J., Liu M., Lin L., Xu X., Zhou Z. Citrus tangeretin reduces the oxidative stress of the myocardium, with the potential for reducing fatigue onset and myocardial damage. J. Funct.Foods. 2019;54:249–253. [Google Scholar]
- Lakshmi A., Subramanian S.P. Tangeretin ameliorates oxidative stress in the renal tissues of rats with experimental breast cancer induced by 7,12-dimethylbenz[a]anthracene. Toxicol. Lett. 2014;229(2):333–348. doi: 10.1016/j.toxlet.2014.06.845. [DOI] [PubMed] [Google Scholar]
- Li C., Wang L., Zhao J., Wei Y., Zhai S., Tan M., Guan K., Huang Z., Chen C. Lonicera rupicola Hook.f.et Thoms flavonoids ameliorated dysregulated inflammatory responses, intestinal barrier, and gut microbiome in ulcerative colitis via PI3K/AKT pathway. Phytomedicine. 2022;104 doi: 10.1016/j.phymed.2022.154284. [DOI] [PubMed] [Google Scholar]
- Li M., Fei Z., Xu T., Song H., Lu B. Acteoside protects against 6-OHDA-induced dopaminergic neuron damage via Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2018;119:6–13. doi: 10.1016/j.fct.2018.06.018. [DOI] [PubMed] [Google Scholar]
- Li S., Ma Y., Ye S., Su Y., Hu D., Xiao F. Endogenous hydrogen sulfide counteracts polystyrene nanoplastics-induced mitochondrial apoptosis and excessive autophagy via regulating Nrf2 and PGC-1α signaling pathway in mouse spermatocyte-derived GC-2spd(ts) cells. Food Chem. Toxicol. 2022;164 doi: 10.1016/j.fct.2022.113071. [DOI] [PubMed] [Google Scholar]
- Liang F., Cao W., Huang Y., Fang Y., Cheng Y., Pan S., Xu X. Isoflavone biochanin A, a novel nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element activator, protects against oxidative damage in HepG2 cells. Biofactors. 2019;45(4):563–574. doi: 10.1002/biof.1514. [DOI] [PubMed] [Google Scholar]
- Liang F., Fang Y., Cao W., Zhang Z., Pan S., Xu X. Attenuation of tert-butyl hydroperoxide (t-BHP)-induced oxidative damage in HepG2 cells by tangeretin: relevance of the Nrf2-ARE and MAPK signaling pathways. J. Agric. Food Chem. 2018;66(25):6317–6325. doi: 10.1021/acs.jafc.8b01875. [DOI] [PubMed] [Google Scholar]
- Liang Y., Zhao J., Zou H., Zhang J., Zhang T. In vitro and in silico evaluation of EGFR targeting activities of curcumin and its derivatives. Food Funct. 2021;12:10667–10675. doi: 10.1016/j.phymed.2021.153736. [DOI] [PubMed] [Google Scholar]
- Liu Q., Hu Y., Cao Y., Song G., Liu X. Chicoric acid ameliorates lipopolysaccharide-induced oxidative stress via promoting the Keap1/Nrf2 transcriptional signaling pathway in BV-2 microglial cells and mouse brain. J. Agric. Food Chem. 2017;65(2):338–347. doi: 10.1021/acs.jafc.6b04873. [DOI] [PubMed] [Google Scholar]
- Lu M., Ji J., Jiang Z., You Q. The Keap1-Nrf2-ARE pathway as apotential preventive and therapeutic target: an update. Med. Res. Rev. 2016;36(5):1–40. doi: 10.1002/med.21396. [DOI] [PubMed] [Google Scholar]
- Lu X., Liu M., Dong H., Miao J., Stagos D., Liu M. Dietary prenylated flavonoid xanthohumol alleviates oxidative damage and accelerates diabetic wound healing via Nrf2 activation. Food Chem. Toxicol. 2022;160 doi: 10.1016/j.fct.2022.112813. [DOI] [PubMed] [Google Scholar]
- Ma D., Wang Z., He Z., Wang Z., Chen Q., Qin F., Zeng M., Chen J. Pine pollen extract alleviates ethanol-induced oxidative stress and apoptosis in HepG2 cells via MAPK signaling. Food Chem. Toxicol. 2023;171 doi: 10.1016/j.fct.2022.113550. [DOI] [PubMed] [Google Scholar]
- Ma L., Wang D., Yu X., Zhou Y. Tangeretin induces cell cycle arrest and apoptosis through upregulation of PTEN expression in glioma cells. Biomed. Pharmacother. 2016;81:491–496. doi: 10.1016/j.biopha.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Müller L., Keuter L., Bücksteeg D., Uebel T., Wilken M., Schürmann L., Behrens M., Humpf H.U., Esselen M. Metabolic conjugation reduces in vitro toxicity of the flavonoid nevadensin. Food Chem. Toxicol. 2022;164 doi: 10.1016/j.fct.2022.113006. [DOI] [PubMed] [Google Scholar]
- Mutlu-Ingok A., Catalkaya G., Capanoglu E., Karbancioglu-Guler F. Antioxidant and antimicrobial activities of fennel, ginger, oregano and thyme essential oils. Food Front. 2021;2:508–518. doi: 10.1002/fft2.77. [DOI] [Google Scholar]
- Peng S., Yao J., Liu Y., Duan D., Zhang X., Fang J. Activation of Nrf2 target enzymes conferring protection against oxidative stress in PC12 cells by ginger principal constituent 6-shogaol. Food Funct. 2015;6(8):2813. doi: 10.1039/C5FO00214A. [DOI] [PubMed] [Google Scholar]
- Puppala E.R., Jain S., Saha P., Rachamalla M., Np S., Yalamarthi S.S., Abubakar M., Chaudhary A., Chamundeswari D., Usn M., Gangasani J.K., Naidu V.G.M. Perillyl alcohol attenuates rheumatoid arthritis via regulating TLR4/NF-κB and Keap1/Nrf2 signaling pathways: a comprehensive study onin-vitro and in-vivo experimental models. Phytomedicine. 2022;97 doi: 10.1016/j.phymed.2022.153926. [DOI] [PubMed] [Google Scholar]
- Qiu W., Zhang X., Pang X., Huang J., Zhou S., Wang R., Tang Z., Su R. Asiatic acid alleviates LPS-induced acute kidney injury in broilers by inhibiting oxidative stress and ferroptosis via activation of the Nrf2 pathway. Food Chem. Toxicol. 2022;170 doi: 10.1016/j.fct.2022.113468. [DOI] [PubMed] [Google Scholar]
- Raza W., Luqman S., Meena A. Prospects of tangeretin as a modulator of cancer targets/pathways. Pharmacol. Res. 2020;161 doi: 10.1016/j.phrs.2020.105202. [DOI] [PubMed] [Google Scholar]
- Ren L., Zhang J., Zhang T. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021;340 doi: 10.1016/j.foodchem.2020.127933. [DOI] [PubMed] [Google Scholar]
- Schijlen E.G., Ric de Vos C.H., van Tunen A.J., Bovy A.G. Modification of flavonoid biosynthesis in crop plants. Phytochemistry. 2004;65:2631–2648. doi: 10.1016/j.phytochem.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Shen B., Wang Y., Cheng J., Peng Y., Zhang Q., Li Z., Zhao L., Deng X., Feng H. Pterostilbene alleviated NAFLD via AMPK/mTOR signaling pathways and autophagy by promoting Nrf2. Phytomedicine. 2022;154561 doi: 10.1016/j.phymed.2022.154561. [DOI] [PubMed] [Google Scholar]
- Shen Y., Fan X., Qu Y., Tang M., Huang Y., Peng Y., Fu Q. Magnoflorine attenuates inflammatory responses in RA by regulating the PI3K/Akt/NF-κB and Keap1-Nrf2/HO-1 signalling pathways in vivo and in vitro. Phytomedicine. 2022;104 doi: 10.1016/j.phymed.2022.154339. [DOI] [PubMed] [Google Scholar]
- Tan Y.Q., Lin F., Ding Y.K., Dai S., Liang Y.X., Zhang Y.S., Li J., Chen H.W. Pharmacological properties of total flavonoids in Scutellaria baicalensis for the treatment of cardiovascular diseases. Phytomedicine. 2022;107 doi: 10.1016/j.phymed.2022.154458. [DOI] [PubMed] [Google Scholar]
- Tsai C.W., Lin C.Y., Wang Y.J. Carnosic acid induces the NAD(P)H: quinone oxidoreductase 1 expression in rat Clone 9 cells through the p38/nuclear factor erythroid-2 related factor 2 pathway. J. Nutr. 2011;141:2119–2125. doi: 10.3945/jn.111.146779. [DOI] [PubMed] [Google Scholar]
- Wang Y., Jin R., Chen J., Cao J., Sun C. Tangeretin maintains antioxidant activity by reducing CUL3 mediated NRF2 ubiquitination. Food Chem. 2021;365 doi: 10.1016/j.foodchem.2021.130470. [DOI] [PubMed] [Google Scholar]
- Wang Y., Meng D., Zhang P., Wang X., Du G., Brennan C., Li S., Ho C.T., Zhao H. Antioxidant protection of nobiletin, 5-demethylnobiletin, tangeretin, and 5-demethyltangeretin from citrus peel in saccharomyces cerevisiae. J. Agric. Food Chem. 2018;66:3155–3166. doi: 10.1021/acs.jafc.8b00509. [DOI] [PubMed] [Google Scholar]
- Wild A.C., Moinova H.R., Mulcahy R.T. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J. Biol. Chem. 1999;274:33627–33636. doi: 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- Yan R., Wang H., Zhu J., Wang T., Nepovimova E., Long M., Li P., Kuca K., Wu W. Procyanidins inhibit zearalenone-induced apoptosis and oxidative stress of porcine testis cells through activation of Nrf2 signaling pathway. Food Chem. Toxicol. 2022;165 doi: 10.1016/j.fct.2022.113061. [DOI] [PubMed] [Google Scholar]
- Yang Y.C., Lii C.K., Wei Y.L., Li C.C., Lu C.Y., Liu K.L., Chen H.W. Docosahexaenoic acid inhibition of inflammation is partially via cross-talk between Nrf2/heme oxygenase 1 and IKK/NF-κB pathways. J. Nutr. Biochem. 2013;24:204–212. doi: 10.1016/j.jnutbio.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Yao J., Ge C., Duan D., Zhang B., Cui X., Peng S., Liu Y., Fang J. Activation of the phase II enzymes for neuroprotection by ginger active constituent 6-dehydrogingerdione in PC12 cells. J. Agric. Food Chem. 2014;62:5507–5518. doi: 10.1021/jf405553v. [DOI] [PubMed] [Google Scholar]
- Zhang J., Pavek P., Kamaraj R., Ren L., Zhang T. Dietary phytochemicals as modulators of human pregnane X receptor. Crit. Rev. Food Sci. 2021;1–23 doi: 10.1080/10408398.2021.1995322. [DOI] [PubMed] [Google Scholar]
- Zhang L., Shi M., Tian M., Wang X., Chen F. Guidelines for absolute quantitative real-time PCR for microbial determination in in vitro gastrointestinal digestion. Food Front. 2020;1:200–204. doi: 10.1002/fft2.31. [DOI] [Google Scholar]
- Zhang T., Zhong S., Li T., Zhang J. Saponins as modulators of nuclear receptors. Crit. Rev. Food Sci. 2020;60:94–107. doi: 10.1018/10408398.2018.1514580. [DOI] [PubMed] [Google Scholar]
- Zou H., Ye H., Kamaraj R., Zhang T., Zhang J., Pavek P. A review on pharmacological activities and synergistic effect of quercetin with small molecule agents. Phytomedicine. 2021;92 doi: 10.1016/j.phymed.2021.153736. [DOI] [PubMed] [Google Scholar]