FIGURE 7.
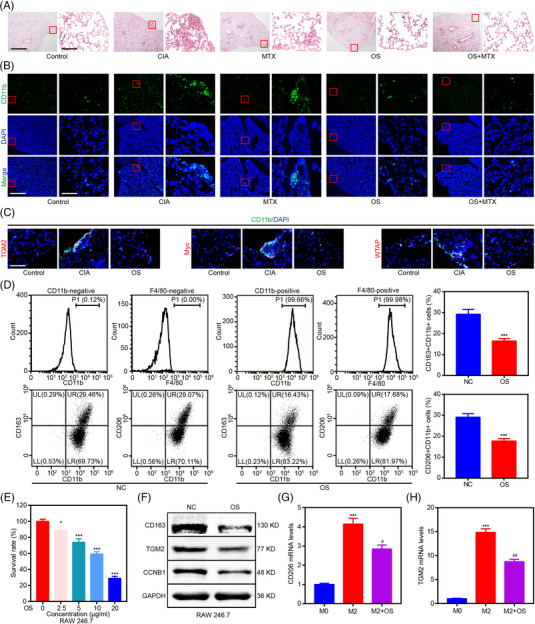
OS suppresses RA‐associated interstitial lung disease (ILD) by downregulating the aggregation of M2 macrophages. (A) Representative images (left scale bar: 125 μm and right scale bar: 12.5 μm) of H&E staining displaying the impact of OS and MTX on the generation of subpleural inflammation in CIA models. (B) Representative images (left scale bar: 125 μm and right scale bar: 12.5 μm) of immunofluorescence staining displaying the impact of OS and MTX on the aggregation of CD11b+ interstitial macrophage in the subpleural inflammation area of the CIA mouse model. (C) Representative images (scale bar: 12.5 μm) of immunofluorescence staining displaying the impact of OS on TGM2, Myc, and WTAP expression of CD11b+ interstitial macrophage in the subpleural inflammation area of the CIA mouse model. (D) Flow cytometry indicating the effect of OS on the percentage of CD163+CD11b+ and CD206+CD11b+ RAW 246.7 cells. (E) RAW 246.7 cells were exposed to OS (0, 2.5, 5, 10, and 20 μg/mL) for 48 h, and cell viability was measured by CCK8 assays. (F) Western blot analyses were applied for elucidating the impact of OS on CCNB1, CD163, and TGM2 protein levels in RAW 246.7 cells. (G, H) qPCR assays were applied to reveal the impact of OS on CD206 (G) and TGM2 (H) in IL4 and IL13‐induced M2 macrophage differentiated from THP‐1. * p < 0.05 and *** p < 0.001 versus control group. # p < 0.05 and ## p < 0.01 versus M2 group.
