Fig. 4. Evaluation of the role of DYRK1A and cAMP signaling in harmine–GLP1R agonist synergy.
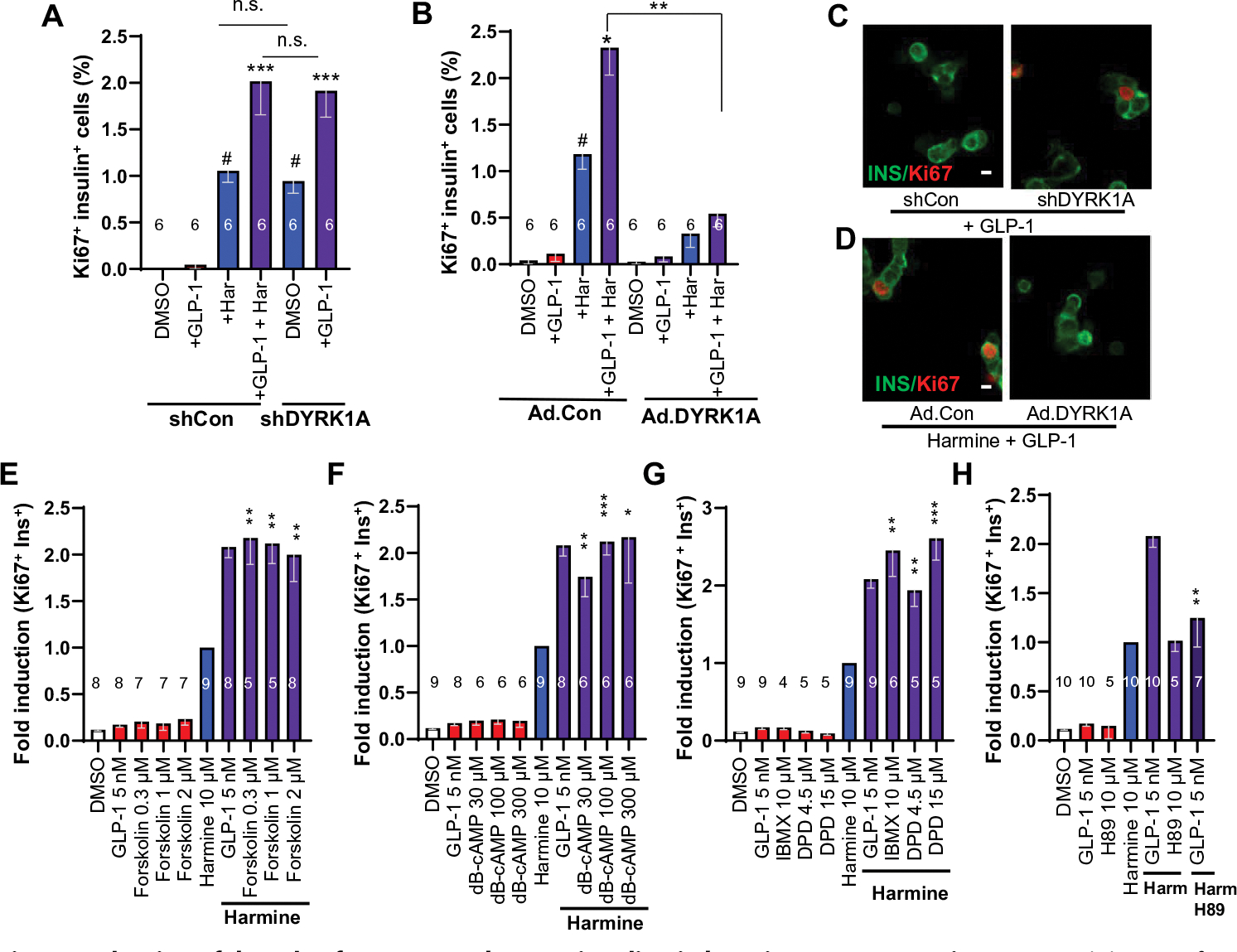
(A) Quantification of Ki67-insulin coimmunolabeling in human islets in response to adenoviral silencing of DYRK1A or harmine (10 μM) and GLP-1 (5 nM). Silencing DYRK1A can replace the effects of harmine in the presence of GLP-1. (B) Quantification of the effects of Ad.DYRK1A overexpression or a control adenovirus (Ad.Con, overexpressing Cre recombinase) in human islets treated with GLP-1 and harmine at various doses. (C) An example of Ki67 immunolabeling in an insulin-positive cell in response to Ad.shRNA silencing of DYRK1A (left), but not with a control, scrambled shRNA adenovirus (left). Scale bar, 10 μm. (D) An example of Ki67-insulin colabeling in β cells of human islets treated with harmine and GLP-1 at the doses in (A) (left) and the absence of proliferation in the presence of Ad.DYRK1A (right). (E to H) Effects on Ki67-insulin colabeling by control vehicle (DMSO), GLP-1, and the following compounds alone or in the presence of harmine: (E) forskolin, (F) dibutyryl-cAMP (dB-cAMP), (G) phosphodiesterase inhibitors isobutylmethylxanthine (IBMX) or dipyridamole (DPD). (H) Effect of PKA inhibitor H89 on Ki67-insulin colabeling on its own and combined with harmine (Harm) and GLP-1. All bars indicate means ± SEM. Numbers of individual human islet donors studied are shown within or above bars. Paired two-tailed t test, #P < 0.01 versus control; *P < 0.05, **P < 0.01, and ***P < 0.001 versus harmine alone. n.s., not significant (P > 0.05).
