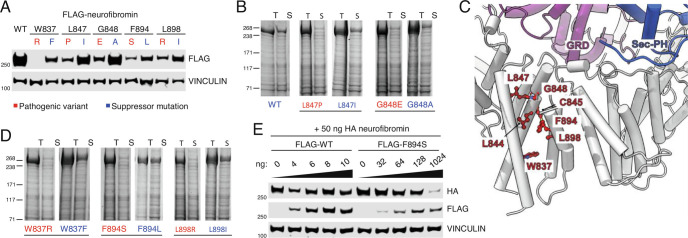
Fig. 4.
Loss of stability in α-helix 34 of the N-terminal HEAT domain can be predicted and rescued with hydrophobic residue substitution. (A) Lysates from 293T NF1−/− cells expressing FLAG-tagged neurofibromin wild type or with pathogenic and alternative ‘suppressor’ residues. (B) Solubility and purification of His6-tagged WT, L847P or L847I and G848E or G848A neurofibromin mutants from baculovirus lysates. T = Total, S = Soluble. (C) Cryo-EM structure reveals the location of additional residues mutated in human patients. These residues (red ball-and-stick) map to the α33-α36 HEAT repeat (gray cartoon) and are juxtaposed to the functionally pivotal HEAT:GRD:Sec-PH interface. GRD is colored purple and Sec-PH is blue. (D) Solubility and purification of W837R, W837F, F894S, F894L, L898R, and L898I mutants as in B. (E) Lysates from 293T NF1−/− where HA-neurofibromin WT was cotransfected with increasing levels of either wild type or mutant (FLAG-tagged).