Abstract
Utilizing the atto-zeptomole sensitivity of UPLC-accelerator mass spectrometry (UPLC-AMS), we previously demonstrated significant first-pass metabolism following escalating (25-250 ng) oral micro-dosing in humans of [14C]-benzo[a]pyrene ([14C]-BaP). The present study examines the potential for supplementation with Brussels sprouts (BS) or 3,3’-diindolylmethane (DIM) to alter plasma levels of [14C]-BaP and metabolites over a 48-h period following micro-dosing with 50 ng (5.4 nCi) [14C]-BaP. Volunteers were dosed with [14C]-BaP following fourteen days on a cruciferous vegetable restricted diet, or the same diet supplemented for seven days with 50 g of BS or 300 mg of BR-DIM® prior to dosing. BS or DIM reduced total [14C] recovered from plasma by 56-67% relative to non-intervention. Dietary supplementation with DIM markedly increased Tmax and reduced Cmax for [14C]-BaP indicative of slower absorption. Both dietary treatments significantly reduced Cmax values of four downstream BaP metabolites, consistent with delaying BaP absorption. Dietary treatments also appeared to reduce the T1/2 and the plasma AUC(0,∞) for Unknown Metabolite C, indicating some effect in accelerating clearance of this metabolite. Toxicokinetic constants for other metabolites followed the pattern for [14C]-BaP (metabolite profiles remained relatively consistent) and non-compartmental analysis did not indicate other significant alterations. Significant amounts of metabolites in plasma were at the bay region of [14C]-BaP irrespective of treatment. Although the number of subjects and large interindividual variation are limitations of this study, it represents the first human trial showing dietary intervention altering toxicokinetics of a defined dose of a known human carcinogen.
Keywords: Benzo[a]pyrene; Brussels sprouts; 3,3’-Diindolylmethane; Human Carcinogen Dosing; Accelerator Mass Spectrometry; Toxicokinetics
Graphical Abstract
1. Introduction
1.1. Benzo[a]pyrene (BaP) formation and carcinogenicity
Polycyclic aromatic hydrocarbons (PAHs) are produced from incomplete combustion of organic materials with common sources being coal, petroleum, wood, and tobacco (ATSDR, 1995; IARC, 1983; 2010, US EPA, 2017). Individual PAHs (BaP and benzo[b]fluoranthene) and PAH mixtures make up three of the top ten chemicals of concern on the ATSDR Substance Priority List and are drivers of remediation at a number of EPA Superfund sites (ATSDR, 2019). BaP is designated as a group 1 known human carcinogen (IARC, 1983) and, based on a large dataset of carcinogenicity in preclinical models, was proposed (suspended as policy in 2019) as the reference PAH for risk assessment of environmental mixtures in the EPA’s Relative Potency Factor (RPF) approach to cancer risk assessment (US EPA, 2010).
Human exposure to BaP is primarily (≥ 90%) through the diet (Bansal and Kim, 2015; Domingo and Nadal, 2015; Kazerouni et al., 2001; Sampaio et al., 2021) and is estimated at 250-700 ng/day for a non-smoking individual in the US (JEFCA, 2005). Serum analysis of BaP in non-smoking Canadian women showed levels of 220 ± 150 pg/mL (Neal et al., 2008). Pleil et al. (2010) found much lower levels in plasma (median of 4 pg/mL) from non-smoking students in the US. In a recent study employing UPLC-accelerator mass spectrometry (UPLC-AMS), a micro-dose of 250 ng of [14C]-BaP produced a Cmax of 8.1 ± 7.6 fg/mL plasma (Maier et al., 2021a). The carcinogenicity of BaP is driven primarily by cytochrome P450 (CYP)-oxygenation (Figure 1) at what is commonly referred to as the “bay region” (Crowell et al., 2014; Shimada, 2006; Shimada and Fujii-Kuriyama, 2004; Uppstad et al., 2010). CYPs in the 1 family first form the 7,8-epoxide (endo or exo) followed by hydrolysis to two trans-7,8-dihydrodiols (7,8-DHD). A second epoxygenation produces four enantiomers of the ultimate carcinogenic 7,8-DHD-9,10-epoxide (DHDE) with the (+)-anti-DHDE being the most potent mutagen (Conney et al., 1994; Shimada and Fujii-Kuriyama, 2004). BaP-DHDE alkylates DNA primarily at the N2 position of guanine and, in the absence of nucleotide excision repair, results in G to T transversion mutations and cancer (Kapitulnik et al., 1978; Osborne et al., 1976; Wood et al., 1977). BaP-DHDE DNA adducts, as well as the 7,8,9,10-tetraol DHDE hydrolysis products, are used as biomarkers of BaP exposure and/or cancer risk (Lodovici et al., 2004; Mensing et al., 2005; Perera et al., 2005; Villalta et al., 2017).
Figure 1.
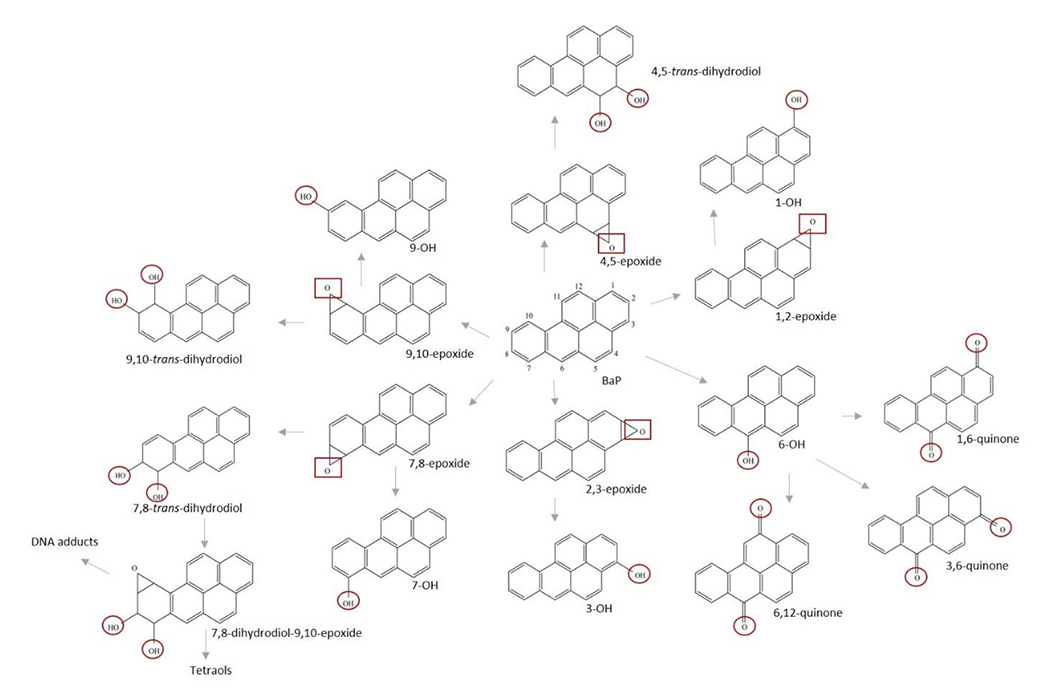
Phase 1 and phase 2 metabolism of benzo[a]pyrene. CYPs 1A1, 1A2, or 1B1 form the epoxide (2 potential isomers) followed by hydrolysis by epoxide hydrolase to dihydrodiols (2 potential isomers). A second epoxygenation produces four enantiomers including 7,8-DHD-9,10-epoxide (DHDE). Quinones are formed through a spontaneous redox reaction following the initial phenol formation. Sites of potential glucuronidation or sulfation represented with circles and potential sites of glutathione conjugation shown with squares.
1.2. Brussels sprouts and DIM as chemopreventive agents
Brussels sprouts are a rich source of the glucosinolate, glucobrassicin, that when hydrolyzed by plant or microbial myrosinase results in production of indole-3-carbinol (I3C) (Hayes et al., 2008; Williams, 2021). I3C is an effective dietary chemopreventive agent in numerous preclinical cancer models and has been the focus of numerous clinical trials (reviewed in Williams, 2021). The efficacy of I3C is due to the production of acid-condensation products in the gut, primarily DIM that is the major, if not sole, I3C acid condensation product detected in systemic circulation (Anderton et al., 2004a ; Bjeldanes et al., 1991; Sepkovic et al., 2001) and does not undergo further acid conjugation reactions (Anderton et al., 2004b; Reed et al., 2008). Urinary levels of DIM are reliable biomarkers for the consumption of Brussels sprouts (Fujioka et al., 2016). Our laboratory has recently characterized mono- and di-hydroxylated DIM metabolites along with their sulfate and glucuronide conjugates in human plasma and urine (Maier et al., 2021b) following the dosing regimen in this study.
1.3. AMS and micro-dosing of humans with carcinogens
The extreme sensitivity of AMS allows for micro-dosing of humans with [14C]-labeled carcinogens with de minimus risk (Brown et al., 2006; Dueker et al., 2011; Enright et al., 2016). AMS has been utilized to assess covalent binding to DNA following dosing of humans with [14C]-labeled PAHs, heterocyclic amines (IQ, MeIQ, PhiP) from cooked meat (Malfatti et al., 1999; Turteltaub et al., 1999), as well as aflatoxin B1 (AFB1) (Cupid et al., 2004). Jubert et al. (2009) demonstrated that co-administration of chlorophyll or chlorophyllin (CHL) was effective in reducing the plasma Cmax and AUC of [14C]-AFB1eq in human participants using total [14C] via AMS-graphite analysis. This study provided mechanistic support for results from a clinical trial in a region of China (Qidong Province) with an extremely high incidence of hepatocellular carcinoma (HCC). Administration of CHL tablets to participants reduced the AFB1-DNA adduct repair product, aflatoxin-N7-guanine, a biomarker of AFB1 exposure and cancer risk (Egner et al., 2001). The development of a UPLC interface for AMS (Thomas et al., 2011) now allows for the determination of the metabolic profile and toxicokinetics of procarcinogens requiring metabolic activation.
1.4. UPLC-AMS in determination of [14C]-BaP toxicokinetics and metabolite profiles
Our initial studies employed graphite AMS (total [14C] only) in the micro-dosing of humans with [14C]-PAHs utilized dibenz[def,p]chrysene (DBC) followed by BaP (Madeen et al., 2015; 2016). UPLC-AMS allowed for characterization of the metabolic profile and toxicokinetics of [14C]-BaP as a function of dose (25-250 ng). It was somewhat surprising to observe the extent of metabolism (90-97% in plasma) following oral administration of [14C]-BaP over this dose range (Maier et al., 2021a). The 7,8,9,10-tetraols, as well as 7,8- and 9,10-DHD and the 9-phenol (bay region metabolites), were the predominant metabolites. In the present study we describe the impact of seven days of supplementation with Brussels sprouts or BR-DIM® on the metabolic profile and pharmacokinetics of a subsequent dose of 50 ng [14C]-BaP.
2. Material and methods
2.1. Human subjects
2.1.1. Recruitment, enrollment, and pre-dosing protocol
This study was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Seven subjects (6 males and 1 female) ranging from 26 to 65 years old with BMIs of 23.8-33.4 (Table 1) were recruited and enrolled under the guidelines of an FDA IND (#117175) and IRBs at both Oregon State University (OSU; protocols 8233 and 8789) and Lawrence Livermore National Laboratory (LLNL; Protocol #2017-008). Exclusion criteria can be found at ClinicalTrials.gov (NCT03802721) and Madeen et al., (2019). Volunteers were given a list of foods from which to abstain (e.g. charcoal-broiled meats and smoked meats or cheeses) for two weeks prior to and during the study. They were also instructed to avoid cruciferous vegetables and certain condiments over this same period (Supplemental Table 1). In order to estimate BaP consumption, all foods and non-water beverages were recorded in a food diary that spanned 3 days prior to and throughout the 48-h study cycle. Volunteers also completed the Arizona Cruciferous Vegetable Food Frequency Questionnaire (ACVFFQ) to assess their usual intake of cruciferous vegetables and condiments in the previous three months (Thomson et al., 2007, 2016) (Supplemental Table 2). A three-week minimum separation between doses was established as a washout period with the requirement that all three doses were administered in less than one year (Table 1).
Table 1.
Demographics of participants and dosing dates
| Volunteer | Age | Sex | BMIa | Race | Hispanic | Dosing Date (& days since initial dose) | ||
|---|---|---|---|---|---|---|---|---|
| Dosed | BS | DIM | ||||||
| BaP021 | 27 | M | 24.3 | White | N | 9-10-19 | (+56) | (+28) |
|
| ||||||||
| BaP022 | 65 | M | 31.8 | White | N | 7-25-18 | (+285) | (+201) |
|
| ||||||||
| BaP025 | 44 | M | 28.0 | White | N | 7-23-18 | (+259) | (+231) |
|
| ||||||||
| BaP028 | 49 | M | 28.0 | White | N | 3-18-19 | (+259) | (+217) |
|
| ||||||||
| BaP031 | 59 | F | 33.4 | White | N | 1-22-19 | (+126) | (+96) |
|
| ||||||||
| BaP041 | 26 | M | 23.8 | Black/AA | N | 11-12-19 | (+104) | (+62) |
|
| ||||||||
| BaP042 | 43 | M | 37.1 | White | N | 10-15-19 | (+105) | (+64) |
BMI listed is at the time of initial dosing of [14C]-BaP. The BMIs at the time of dosing following Brussels sprouts and DIM were BaP021, 24.8, 24.7; BaP022, 32.8, 32.6; BaP025, 26.0, 26.3; BaP028, 28.5, 28.0; BaP031, 33.6, 33.9; BaP041, 23.8, 23.0; BaP042, 37.7, 36.0.
2.1.2. Dietary intervention with brussels sprouts or DIM
Brussels sprouts were provided precooked, frozen, in seven individually vacuum-sealed packets. Volunteers consumed either brussels sprouts (50 grams, thawed, warmed, and seasoned as desired) or two capsules (300 mg total) of bioResponse DIM® 150 (45.3 mg DIM/capsule) daily with dinner for seven days prior to the 48-h study cycle. based on self-report diaries and empty return vials, compliance was 100%. Volunteers also consumed two BR-DIM® capsules at the start of the 48-h toxicokinetic study during the DIM arm of the study. Prior to consumption of BR-DIM® capsules and/or [14C]-BaP, 25 mL of blood and spot urine were collected as a baseline for background DIM. This plasma was also used to obtain background levels of 64 different PAHs by GC-MS/MS as previously described (Maier et al., 2021a). Subjects fasted overnight (10-12 h) prior to the initiation of the 48-h study of [14C]-BaP toxicokinetics.
2.1.3. Micro-dosing with [14C]-BaP, timed blood collection, and preparation of plasma
Participants were given a food-grade capsule containing 50 ng of [14C]-BaP (5.4 nCi) with 200-250 mL water. Quality control with respect to the purity of [14C]-BaP as well as preparation and quality control of the capsule was approved by FDA (IND117175) and has been previously described (Hummel et al., 2018). Fasting continued for 2 h following dosing after which participants were provided a breakfast. Blood was drawn into heparinized tubes prior to the study (time 0, 25 mL) and 0.25, 0.5, 1, 1.5, 2, 3, and 4 h post-dose (10 mL) using an in-dwelling catheter. Later blood collections at 8, 24, and 48 h post-dose (10 mL) were done by straight needle stick. Blood was centrifuged (rcf‘~1000 for 10 min) immediately following the draw and held at 4°C until extraction.
2.2. Extraction of plasma and UPLC-AMS at Lawrence Livermore National Laboratory (LLNL)
2.2.1. Plasma extraction
All plasma was extracted within 2 h of collection with established methods (Crowell et al., 2011; Hummel et al., 2018). Plasma aliquots (1.5 mL) were acidified and vortexed thrice with ethyl acetate (1.5 mL) containing K2SO4. Ethyl acetate extracts of each sample were pooled, evaporated to dryness under nitrogen, reconstituted with 100 μL ethyl acetate and shipped on dry ice to the BioAMS Center at LLNL. Plasma aliquots used to determine pre-dosing levels of PAHs were stored at −80°C until GC-MS/MS analysis by the Analytical Chemistry Core of the Oregon State University Superfund Research Program (Maier et al., 2021a).
2.2.2. UPLC-AMS separation, detection, and identification
Just prior to UPLC-AMS analysis, samples were taken to dryness under vacuum and reconstituted with 50 μL acetonitrile. Samples were maintained in the autosampler of a Waters Acquity UPLC H-Class System at 20°C, the column at 28°C, and injection volume set at 10 μL. Elution of [14C]-BaP and metabolites was achieved over 20 min with a Waters Acquity UPLC BEH C18 1.7 μm 2.1 x 50 mm column equipped with a guard column (Vanguard HSS PFP 1.8 μm 2.1 x 5 mm) and a gradient of 0.3% formic acid in water (A) and PEG-free acetonitrile (B) at 0.25 mL/min (0.0 min, 70:30 A:B; 0.1 min, 60:40; 10.0 min, 54:46; 10.1 min, 43:57; 14.3 min, to 16.3 min, 0:100; 16.4 min to 20 min 70:30). [14C]-BaP metabolites were identified by retention time using analytical standards obtained from Oregon State University Superfund Program PAH repository (http://limsweb.science.oregonstate.edu/). Elution times of metabolites and parent compound included in the analytical standard are given in Supplemental Table 3. The eluate first went through a PDA detector and then was split; 0.12 mL/min (48%) with deposition onto a moving wire interface for AMS and the remainder sent to waste. The eluate was carried by a periodically indented nickel wire through a drying oven to evaporate solvent followed by a combustion oven to convert analytes to [14C]-CO2, allowing transfer to the CO2-gas-accepting ion source of the 250 kV AMS (Ognibene et al., 2015; Thomas et al., 2011). 13C and 14C have known isotope ratios allowing for quantification of [14C]-BaP and metabolites. Accuracy (1-3%), precision (expressed as a coefficient of variation of 1-6%), and sensitivity expressed as limit of detection (LOD) of 1 attomole of [14C] (0.58 fg for [14C]-BaP) have been established (Keck et al., 2010; Ognibene et al., 2019). The LOQ (0.60 fg/mL) was established with plasma blanks from each individual and at elution times for each compound quantified (Maier et al., 2021a).
AMS measurements of 12C, 13C, and 14C in biological samples vary by source article, individual, and sample type (Keck et al., 2010; Salehpour et al., 2008). AMS counts individual 14C atoms in relation to 12C and/or 13C ion currents with extremely high sensitivity, and any variation in background signal impacts the degree of confidence in LOQ. To increase confidence, a matrix-matched LOQ was established using the standard deviation of the signal in the samples from each participant taken just prior to dosing with [14C]-BaP. By time-matching signal to noise ratios from blanks and study samples, the LOQ is matched to individual peaks of interest and compared to background noise. This results in an LOQ equal to 10-x the signal standard deviation in blanks (at the respective elution times).
Due to the unavailability of radiolabeled BaP metabolite standards, chromatography and metabolite identification cannot be confirmed with AMS directly. In order to use BaP metabolite standards with known retention times to identify metabolites, the UPLC eluate went to a PDA detector and a moving wire interface for AMS analysis; however, not all peaks seen using UPLC-AMS could be identified using the analytical standard mix. Unknown A eluted at the beginning of the chromatography run (0.48 min) and is likely conjugated metabolites. BaP phenols, dihydrodiols, dihydrodiol-epoxides, and quinones are all subject to conjugation (glucuronides, sulfates, and glutathione) and would be the most polar BaP metabolites in plasma. Based on retention times, Unknowns B (3.16 min) and C (4.85 min) are possibly additional dihydrodiols. Unknown D (5.39 min) is either a late eluting dihydrodiol or a quinone, while Unknown E (9.18) is likely a quinone. The peak identified as the 3-phenol may also contain small amounts of the 7- and/or 1-OH-BaP phenols as these minor hydroxylated metabolites tend to co-elute. As reported previously (Maier et al., 2021a) no PAHs, including BaP (LOD, 240 pg/mL), classified as known or probable human carcinogens, were detected in plasma from any participant by GC-MS/MS prior to micro-dosing with [14C]-BaP.
2.3. Toxicokinetic analysis
Time-course concentrations of [14C]-BaP, individual BaP metabolites, and a sum of BaP metabolites were evaluated using non-compartmental analyses. Area under the curve (AUC) of concentrations in plasma from time zero to the last measured time point and extrapolated to infinity (using last 3 time points) were calculated using the trapezoidal rule (Gibaldi and Perrier 1982). Mean residence times were calculated as a ratio of AUC under the 1st moment curve extrapolated to infinity to AUC extrapolated to infinity. Non-compartmental half-lives were calculated as the product of mean resident times with the natural log of two. Linear regression models were used to evaluate impact of BMI on non-compartmental pharmacokinetic parameters from participants receiving [14C]-BaP only. We used linear mixed effects models to examine the relationship between non-compartmental BaP and BaP metabolite pharmacokinetic parameters maximum concentration (Cmax), AUC to last time point (AUC(0,48)), AUC to infinity (AUC(0,∞)), half-life (T1/2), clearance (Cl), and volume of distribution (Vd) and effects of DIM and Brussels sprouts supplementation. In order to account for repeated measures, linear mixed effects models set treatment effects (i.e. BaP only, DIM, or Brussels sprouts) as fixed effects with random intercepts for subjects. P-values were obtained using the likelihood ratio tests of the full model with the effect in question against the model without the effect in question. The non-parametric Friedman Rank Sum Test was used to evaluate treatment effects on Tmax (Supplemental Table 4). We analyzed non-detect data using two assumptions consistent with maximum upper and lower biases. First, we assumed non-detects were 0, providing a maximum lower bias to the data. Second, we assumed non-detects were approximate limits of detection (0.6 fg BaP/mL), providing the maximal upper bias. We acknowledge that the reality is somewhere in between these two assumptions. Major overall conclusions did not change when using both assumptions, and we reported p-values assuming non-detects were 0 in the text and provide both p-values in the supplemental information (Supplemental Table 5). Software used to statistically analyze data was “R: A language and environment for statistical computing” Version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). Linear mixed effects models were fit using the lme4 package (Bates et al. 2015).
3. Results
3.1. Dietary crucifer consumption by participants
Volunteers were instructed to restrict intake of cruciferous vegetables (Supplemental Table 1) for two weeks prior to administration of supplements and during the 48 h period following [14C]-BaP dosing. In addition, the Arizona Cruciferous Vegetable Food Frequency Questionnaire (Thomson et al., 2007) was conducted to estimate crucifer consumption over the three-month period preceding the study (Supplemental Table 2). A wide range of cruciferous vegetable consumption (64 ± 40 g/day with a range of 4-127 g/day) was observed for the participants in this study. For comparison, the average daily consumption in the US is 25-30 g/day (IARC, 2004). For participants in this study, total glucosinolate consumption is estimated at 54 ± 34 mg/day (range 8-105 mg/day) based on reported crucifer intake. The US daily average intake is estimate at 21-25 mg/day (IARC, 2004).
3.2. Consumption of BaP estimated from diet diaries
Volunteers were provided a list of foods highest in BaP to avoid for two weeks prior to initiation of the study and throughout the study as previously described (Maier et al., 2021b). Daily dietary consumption of BaP was estimated over three days pre-study as well as during the study. The average low estimates for the three pre-dosing periods were 206 ± 79, 201 ± 52, and 187 ± 114 ng/day with a range of 75-384 ng/day (Table 2). The high intake estimates for the three pre-dosing periods were 256 ± 83, 283 ± 82, and 262 ± 170 ng/day with a range of 80-556 ng/day. These values are near or below the range for the daily dietary intake for a non-smoking individual in the U.S of 250-700 ng (JEFCA, 2005). The low and high estimates of daily BaP intake for the non-supplemented group during the 2-day study was 408 ± 287 ng and 514 ± 378 ng, respectively. During the study period, following a week of Brussels sprout supplementation, the low and high BaP daily intake estimates were 253 ± 134 ng and 324 ± 155 ng, respectively. Following a week of DIM supplements the low and high BaP daily intake estimates were 201 ± 83 and 273 ± 113 ng, respectively (Table 2).
Table 2.
Estimated average low and high dietary BaP consumption prior to and during study as estimated by self-reported food logs (ng/day)a
| Volunteer | Pre-dosingb | Post-dosingb | Pre-dosing Brussels sproutsc | Post-Brussels sprouts after dosingc | Pre-dosing DIMd | Post-DIM after dosingd | Average ± SD Low | Average ± SD High | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | H | L | H | L | H | L | H | L | H | L | H | |||
| 021 | 189 | 200 | 130 | 165 | 151 | 179 | 167 | 173 | 78 | 80 | 121 | 169 | 139±39 | 161±42 |
| 022 | 138 | 208 | 247 | 325 | 154 | 196 | 462 | 538 | 107 | 168 | 128 | 187 | 206±134 | 270±142 |
| 025 | 331 | 393 | 274 | 368 | .220 | 305 | 151 | 228 | 194 | 356 | 315 | 438 | 248±071 | 348±73 |
| 028 | 141 | 222 | 783 | 1215 | 273 | 389 | 208 | 449 | 384 | 556 | 291 | 408 | 347±229 | 540±348 |
| 031 | 208 | 289 | 829 | 876 | 253 | 381 | 128 | 161 | 276 | 356 | 244 | 312 | 323±253 | 396±247 |
| 041 | 148 | 178 | 572 | 663 | 211 | 283 | 420 | 462 | 75 | 108 | 121 | 190 | 258±196 | 314±210 |
| 042 | 170 | 193 | 343 | 387 | 147 | 250 | 236 | 258 | 195 | 207 | 184 | 205 | 213±70 | 250±72 |
| Average ± SD | 206 79 |
256 83 |
408 287 |
514 378 |
201 52 |
283 82 |
253 134 |
324 155 |
187 114 |
262 170 |
201 83 |
273 113 |
||
The source of the low (L) and high (H) estimates in foods was taken from Jakszyn et al., 2004; Kazerouniac, et al., 2001; EFSA, 2008; Wu and Yu, 2012; Zelinkova and Wenzl, 2015
The “pre-“ is the 3 days prior to [14C]-BaP dosing and the estimates for columns labeled “post-“ are from the 2-days post dosing
Non-supplemented
Brussels sprout arm of the study
The DIM arm of the study
3.3. Background levels of PAHs in plasma
As previously described, GC-MS/MS analysis capable of quantifying 64 PAHs was employed to analyze levels in plasma for all individuals prior to each micro-dosing with [14C]-BaP. A complete description of the method and results can be found in Maier et al. (2021a) and results for this study given in Table 3. Naphthalene was present at quantifiable levels in plasma from all individuals over all three arms of the study. The levels and frequency of naphthalene and alkylated naphthalenes were in the range and frequency of our previous study, as was fluorene (Maier et al. 2021a). Phenanthrene, pyrene, and 1-methylpyrene were similar with respect to previous plasma levels, but the percentage of detects for both phenanthrene and pyrene was higher in this study (50% versus 18% of samples, and 68% compared to 25%, respectively). As before, the PAH found at the highest level was perylene (18-24 ng/mL), but it was only present in 3/22 samples. Retene, a PAH especially abundant in smoke from wildfires, was present in all but one sample at levels of 5.3 ± 2.2 ng/mL (range <LOQ-11.8 ng/mL) compared to our previous study (8.8 ± 10.7 with 100% frequency). In addition to the high levels, frequency of detection and presence in wildfire smoke, retene is of interest given it appears effective in activation of AHR signaling and AHR-dependent toxicity in zebrafish (Geier et al., 2018). Retene is not capable of being metabolized to a DHDE, but human HepG2 cells produce toxic mono- and di-ortho quinones (redox cycling), probably through CYP1A1 and epoxide hydrolase production of DHDs followed by aldoketo reductase oxidation to catechols (Huang et al., 2017).
Table 3.
Background levels of PAHs for each subject and treatment groupa
| Subject/Following | Naphthaleneb,c | 1-Methylnaphthalene | 2-Methylnaphthalene | 1,6- and 2,6-Dimethylnaphthalene | 2-Ethylnaphthalene | Phenanthrene | 2-Methylpheneanthrene | Fluorene | Pyrene | 1-Methylpyrene | Perylene | Retene |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 021/BaP only | 1.44 | - | 0.20 | - | - | 0.64 | - | 0.39 | 0.60 | 0.72 | - | 9.19 |
| 021/BS | 0.63 | 0.16 | 0.39 | - | - | 0.57 | 0.51 | 0.16 | 0.71 | 0.64 | - | 6.68 |
| 021/DIM | 0.59 | 0.19 | 0.55 | 0.45 | 0.25 | 0.44 | - | 0.12 | 0.67 | 0.48 | - | 5.90 |
|
| ||||||||||||
| 022/BaP only | 1.45 | - | 0.99 | - | - | - | - | 0.53 | - | 0.32 | - | 3.05 |
| 022/BS | 0.23 | - | - | - | - | - | - | - | 1.20 | - | 19.2 | 6.85 |
| 022/DIM | 0.39 | - | - | - | - | 0.34 | - | - | - | 0.77 | - | 11.8 |
|
| ||||||||||||
| 025/BaP only | 0.38 | - | - | - | - | - | - | 0.40 | - | - | - | 1.01 |
| 025/BS | 0.14 | - | - | - | - | - | - | - | 0.16 | - | - | - |
| 025/DIM | 0.48 | - | - | - | - | - | - | - | - | 0.38 | - | 4.62 |
|
| ||||||||||||
| 028/BaP only | 0.67 | - | - | - | - | - | - | - | - | 0.34 | - | 4.64 |
| 028/BS | 0.45 | 0.17 | 0.63 | 0.51 | - | - | 0.30 | 0.14 | 0.44 | 0.43 | - | 4.22 |
| 028/DIM | 0.43 | - | 0.65 | 0.55 | - | 0.38 | 0.42 | 0.15 | 0.62 | 0.49 | - | 5.66 |
|
| ||||||||||||
| 031/BaP only | 0.88 | - | - | - | - | - | - | - | - | 0.35 | - | 4.38 |
| 031/BS | 0.20 | - | - | - | - | - | - | - | 0.87 | - | 18.1 | 5.50 |
| 031/DIM | 0.26 | - | - | - | - | - | - | - | 0.62 | - | 24.1 | 6.00 |
|
| ||||||||||||
| 041/BaP only | 0.58 | 0.20 | 0.28 | 0.46 | - | 0.38 | 0.47 | 0.16 | 0.52 | 0.55 | - | 5.80 |
| 041/BS | 0.32 | - | 0.32 | 0.43 | - | 0.58 | 0.62 | 0.16 | 0.88 | 0.51 | - | 5.34 |
| 041/DIM | 0.34 | - | 0.24 | - | - | 0.55 | 0.51 | 0.18 | 0.93 | 0.58 | - | 6.32 |
|
| ||||||||||||
| 042/BaP only | 0.37 | - | 0.55 | 0.53 | - | 0.55 | 0.43 | 0.14 | 0.58 | 0.45 | - | 4.10 |
| 042/BS | 0.37 | - | 1.08 | - | - | 0.97 | 0.30 | - | 0.59 | 0.40 | - | 3.10 |
| 042/DIM | 0.31 | 0.14 | 0.57 | 0.52 | - | 0.69 | 0.48 | 0.17 | 0.91 | 0.57 | - | 5.60 |
64 PAHs were analyzed in plasm from blood taken just prior to the dosing of [14C]-BaP; BaP only, BS and DIM are the non-supplemented, Brussels sprouts, and DIM arms of the study
Results are in ng/mL of plasma
Only the PAHs above the LOQ for at least one occurrence are tabulated and no BaP or any other PAH classified by IARC as a known or probable human carcinogen was present above the LOQ
3.4. Toxicokinetics of [14C] BaP in plasma
Following an oral dose of 50 ng [14C]-BaP, the seven participants displayed similar time course patterns of [14C]-BaP in plasma (Figure 2). Maximum concentrations (Cmax) of [14C]-BaP in blood peaked at 3.69 ± 4.12 fg/mL (mean ± SD) at a median time of 1.25 h. [14C]-BaP T1/2 in blood was 3.69 ± 3.80 h (mean ± SD) and only one blood sample had detectable BaP at 48 h. No statistically significant relationships among Cmax, AUC(0,48 h), AUC(0,∞), T1/2, clearance, or Vd were observed as a function of BMI or age (p ≥ 0.35).
Figure 2.
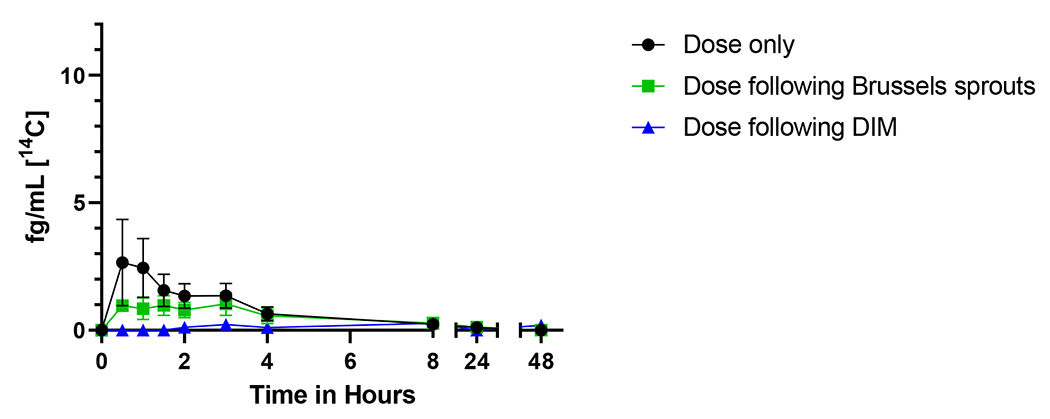
Time course of [14C]-benzo[a]pyrene in plasma from seven individuals over 48 h. The mean plasma levels (fg/mL) ± SE of [14C] for parent BaP is shown with no supplementation (black circles), one week of Brussels sprouts (green squares), or one week of DIM (blue triangles).
Supplementation for one week with DIM significantly altered [14C]-BaP toxicokinetics in plasma of human participants. Treatment of DIM significantly reduced [14C]-BaP Cmax 5-fold (p = 0.048) and significantly increased Tmax 9-fold (p = 0.033) compared to no treatment (Figure 2, Table 4). Effects of Brussel sprouts on BaP Cmax and Tmax were not statically significant. BaP AUCs, T1/2, Vd, and clearance were not significantly affected by dietary treatments (p ≥ 0.29). These changes provide evidence that DIM treatment delayed BaP absorption (reduced Cmax and increased Tmax).
Table 4.
Summary of pharmacokinetic constants for [14C]-BaPa
| Parameterb | BaP only | Dose BS |
DIM |
|---|---|---|---|
| Cmax (fg BaP/mL)c | 3.69 ± 4.12 | 1.67 ± 1.03 | 0.68 ± 0.51 |
| Tmax (h)d | 1.58 ± 1.16 | 1.14 ± 0.90 | 14.00 ± 19.17 |
| AUC (fg BaP/mL×h) | 10.93 ± 9.19 | 9.34 ± 10.02 | 5.51 ± 6.17 |
| AUC(0,∞) (fg BaP/mL×h) | 10.93 ± 9.19 | 9.34 ± 10.02 | 5.49 ± 6.15 |
| T1/2 (h) | 3.69 ± 3.80 | 3.86 ± 4.62 | 9.75 ± 13.25 |
| CI (L/h)e | 14836.59 ± 26620.71 | 51409.16 ± 80206.38 | 22025.01 ± 33131.07 |
| Vd (L)e | 48883.70 ± 78019.06 | 54224.57 ± 44889.28 | 106380.48 ± 88815.96 |
The values shown are the mean ± standard deviation of all participants
Toxicokinetic measures that were significantly different are shown in bold as determined by the Freidman test (Tmax only), or the likelihood ratio tests of mixed effect models.
DIM significantly reduced [14C]-BaP Cmax (p = 0.048).
DIM significantly increased [14C]-BaP Tmax (p = 0.034).
Note: BaP was through oral administration and bioavailability has not been determined in this study.
3.5. Toxicokinetics of [14C] BaP metabolites in plasma over 48 hours
The toxicokinetics for the sum of all [14C]-BaP metabolites in plasma (Figure 3) followed concentric patterns in all three arms of the study (Figure 4–6, Table 4). Although not statistically significant, Cmax of the total metabolites from [14C]-BaP and summed bay region metabolites from plasma of non-supplemented subjects appeared higher than those given dietary supplements (p = 0.057 and 0.065, respectively). Total [14C]-BaP metabolites made up 96.1-100% of the total [14C] in plasma even at the earliest sampling time, indicating rapid and extensive metabolism of BaP (Figure 3). As in our previous study (Maier, 2021a) the Tmax ranged between 1-2 h and little or no [14C] remained in plasma 24 - 48 h post-dose. Total metabolite AUCs, T1/2, and Tmax values were not significantly affected by dietary treatment (p ≥ 0.11).
Figure 3.
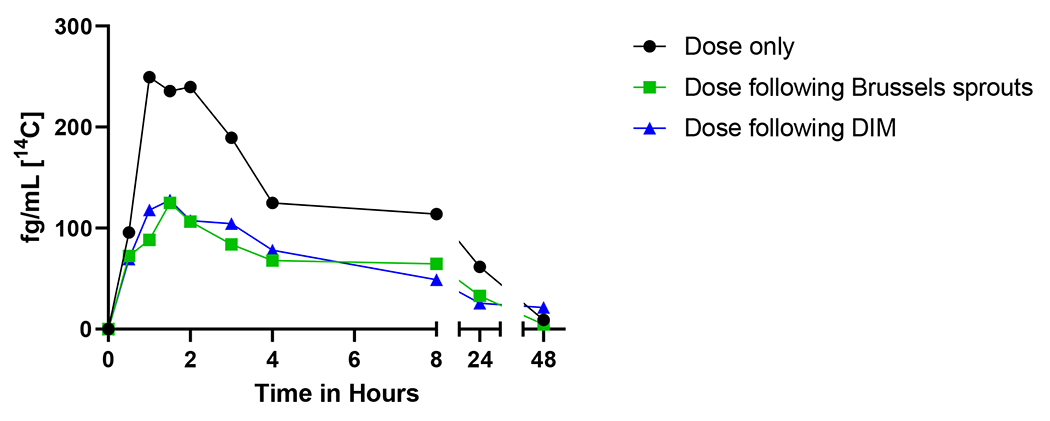
Time course of the sum of total metabolites over 48 h. The total [14C] plasma levels (fg/mL) of the combined twelve metabolites from seven participants is shown with no supplementation (black circles), one week of Brussels sprouts (green squares), or one week of DIM (blue triangles). Standard error of the sum too low to be visualized.
Figure 4.
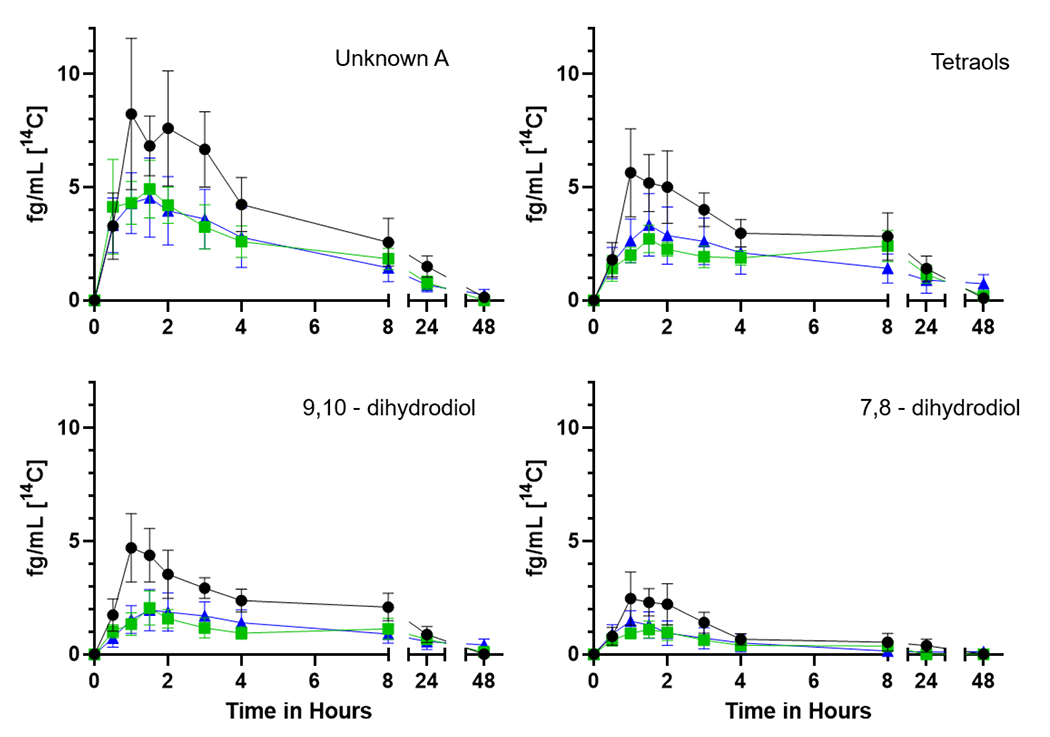
Time course of the [14C] plasma metabolites unknown A, tetraols, 9,10- and 7,8-dihydrodiols over 48 h from seven participants. The mean plasma levels (fg/mL) ± SE of [14C] for the (upper left) unknown peak A (likely conjugates), (upper right) mix of 7,8,9,10-tetraol isomers, (lower left) 9,10- and (lower right) 7,8-dihydrodiols are shown with no supplementation (black circles), one week of Brussels sprouts (green squares), or one week of DIM (blue triangles).
Figure 6.
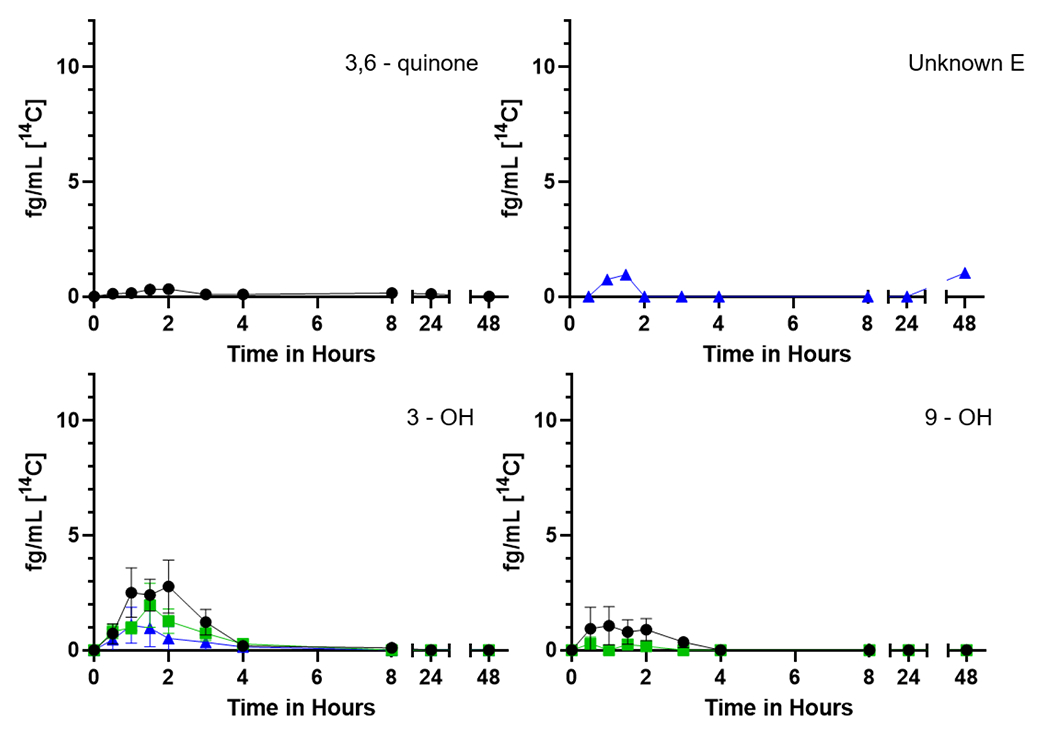
Time course of the [14C] representing individual plasma 3,6-quinone, unknown E, and phenol metabolites over 48 h from seven participants. The mean plasma levels (fg/mL) ± SE of [14C] for the (upper left) 3,6-quinone, (upper right) unknown E, (lower left) 3-phenol and (lower right) 9-phenol are shown with no supplementation (black circles), one week of Brussels sprouts (green squares), or one week of DIM (blue triangles).
Early eluting peaks, indicative of more water-soluble metabolites, predominate (Figure 4). We have posited that Unknown A (no reference standard available for any of the metabolites labeled as unknowns) likely represents highly polar conjugates such as glucuronides, sulfates, or glutathione (Maier et al. 2021a). Tetraols are a mix of isomers of 7,8,9,10-tetraol formed following hydrolysis of the four enantiomers of the carcinogenic metabolite BaP-7,8-DHD-9,10-E. The 9,10- and 7,8-DHDs are the next to elute flanked by 3 unknowns (Figure 5) which may be a diol (unknown B), additional diols or quinones (unknowns C and D) followed by the 1,6- and 3,6-quinones, the 9- and 3-phenols (Figure 6) and finally parent BaP (Supplemental Table 3).
Figure 5.
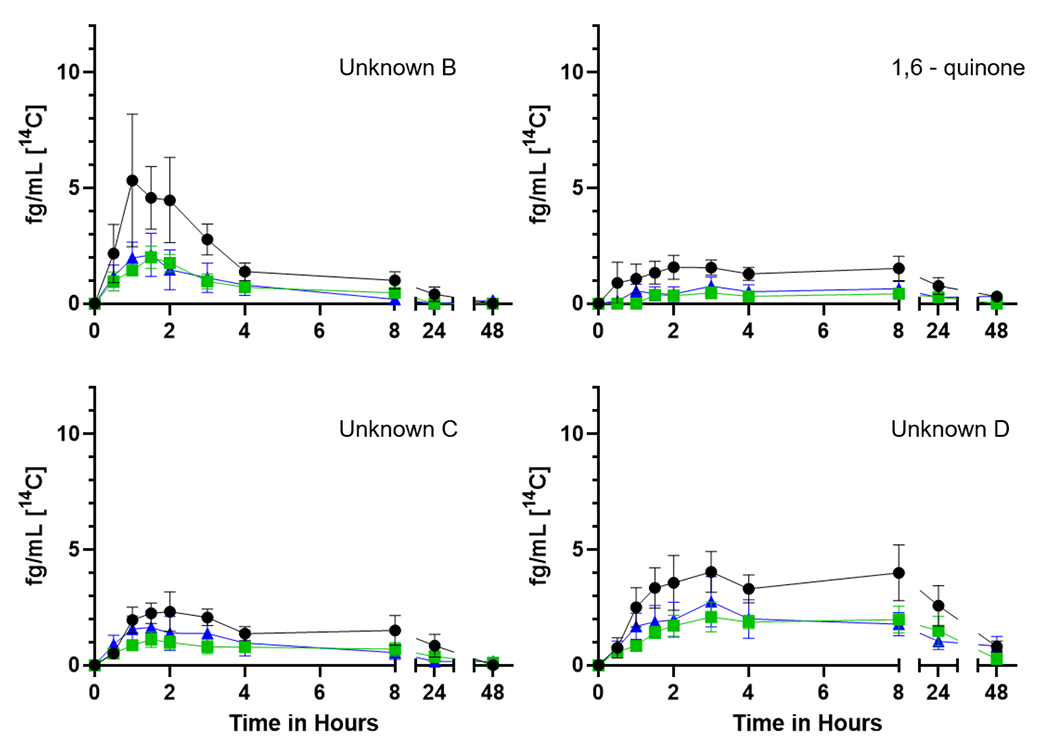
Time course of the [14C] representing individual plasma metabolites unknown B, C and D as well as 1,6-quinone over 48 h from seven participants. The mean plasma levels (fg/mL) ± SE of [14C] for the (upper left) unknown peak B, 1,6-quinone (upper right) unknown peak C, and (lower center) unknown D are shown with no supplementation (black circles), one week of Brussels sprouts (green squares), or one week of DIM (blue triangles).
Dietary treatments caused significant changes to a number of non-compartmental pharmacokinetic parameters of certain metabolites. Cmax of 4 different metabolites (Unknown A, 9,10-DHD, Unknown D, and 3,6-dione) were significantly reduced by DIM and/or Brussel sprout treatments ((p ≤ 0.033, Table 5). These observed downstream effects are consistent with dietary treatments delaying BaP absorption as observed with DIM effects on BaP concentrations in plasma. Interestingly, 3,6-dione was not detected following either dietary supplement. Unknown metabolite E was only detected following DIM treatment. 9-phenol was not detected following DIM treatment. Brussels sprouts significantly reduced the T1/2 of Unknown C (p = 0.022), and while not significant, DIM trended in a similar pattern (p = 0.17). Both dietary treatments significantly reduced the AUC(0,∞) of Unknown C (p = 0.015,). Brussels sprouts significantly reduced AUC and AUC(0,∞) of Unknown D (p ≤ 0.037, Table 5). These observed patterns of non-compartmental toxicokinetic parameters of metabolites are consistent with dietary treatment effects on [14C]-BaP absorption with resulting downstream impacts on metabolites.
Table 5.
Summary of pharmacokinetic constants for [14C]-BaP individual metabolitesa
| Compound | Following | Cmaxd (fg BaP/mL) | Tmax (h) | AUCg (fg BaP/mL×h) | AUC(0,∞)e (fg BaP/mL×h) | T1/2f (h) |
|---|---|---|---|---|---|---|
| Unknown A | BaP only | 10.63 ± 8.59 | 1.5 ± 0.7 | 88.26 ± 67.48 | 99.38 ± 70.48 | 14.46 ± 17.83 |
| BS | 6.28 ± 4.85 | 1.3 ± 1.0 | 47.63 ± 28.47 | 47.63 ± 28.47 | 6.52 ± 3.89 | |
| DIM | 5.07 ± 4.24 | 1.4 ± 0.8 | 49.33 ± 37.09 | 49.31 ± 37.10 | 9.26 ± 8.65 | |
|
| ||||||
| Tetraols | BaP only | 6.89 ± 5.06 | 1.9 ± 1.1 | 78.31 ± 65.08 | 80.88 ± 65.40 | 8.93 ± 5.23 |
| BS | 3.65 ± 1.10 | 7.8 ± 7.8 | 58.50 ± 30.23 | 52.01 ± 27.26 | 7.95 ± 3.26 | |
| DIM | 4.13 ± 3.26 | 18.1 ± 22.0 | 54.06 ± 50.93 | 53.23 ± 59.27 | 10.68 ± 10.77 | |
|
| ||||||
| 9,10-dihydrodiol | BaP only | 5.61 ± 4.10 | 2.1 ± 1.3 | 54.86 ± 41.68 | 54.86 ± 41.68 | 6.04 ± 3.06 |
| BS | 2.22 ± 0.35 | 2.3 ± 2.7 | 29.56 ± 17.48 | 29.55 ± 17.47 | 10.00 ± 6.14 | |
| DIM | 2.64 ± 2.04 | 18.2 ± 21.9 | 33.57 ± 30.42 | 30.94 ± 32.46 | 9.78 ± 11.45 | |
|
| ||||||
| Unknown B | BaP only | 6.68 ± 7.38 | 1.4 ± 0.3 | 32.98 ± 37.79 | 32.98 ± 37.79 | 3.98 ± 2.69 |
| BS | 2.15 ± 0.88 | 1.4 ± 0.8 | 9.64 ± 7.01 | 9.64 ± 7.01 | 2.62 ± 1.50 | |
| DIM | 2.46 ± 2.25 | 4.7 ± 9.2 | 9.98 ± 12.84 | 9.97 ± 12.84 | 5.52 ± 10.42 | |
|
| ||||||
| 7,8-dihydrodiol | BaP only | 3.06 ± 2.94 | 1.6 ± 0.7 | 20.13 ± 30.87 | 20.13 ± 30.87 | 3.66 ± 3.87 |
| BS | 1.51 ± 0.60 | 1.2 ± 0.5 | 6.47 ± 5.55 | 6.47 ± 5.55 | 2.73 ± 1.74 | |
| DIM | 1.69 ± 1.46 | 1.1 ± 0.4 | 8.96 ± 10.31 | 8.95 ± 10.30 | 8.34 ± 11.65 | |
|
| ||||||
| 1,6-quinone | BaP only | 2.82 ± 2.08 | 2.9 ± 2.5 | 41.25 ± 47.16 | 41.25 ± 47.16 | 5.80 ± 3.94 |
| BS | 1.45 ± 0.39 | 6.9 ± 8.1 | 18.14 ± 13.02 | 18.14 ± 13.01 | 7.26 ± 7.10 | |
| DIM | 1.95 ± 1.79 | 9.2 ± 19.0 | 17.01 ± 18.35 | 17.00 ± 18.35 | 8.38 ± 10.87 | |
|
| ||||||
| Unknown C | BaP only | 5.16 ± 3.43 | 4.4 ± 2.5 | 118.92 ± 89.90 | 160.14 ± 107.74 | 26.26 ± 23.18 |
| BS | 3.12 ± 1.57 | 10.2 ± 9.8 | 59.86 ± 34.51 | 55.77 ± 35.89 | 8.39 ± 4.64 | |
| DIM | 3.24 ± 2.69 | 18.4 ± 21.8 | 59.17 ± 33.64 | 63.91 ± 47.84 | 13.84 ± 7.06 | |
|
| ||||||
| Unknown D | BaP only | 2.56 ± 2.07 | 4.3 ± 3.1 | 41.17 ± 36.08 | 43.11 ± 44.22 | 10.47 ± 3.86 |
| BS | 0.77 ± 0.64 | 13.4 ± 9.9 | 11.09 ± 9.74 | 11.09 ± 9.74 | 9.56 ± 6.20 | |
| DIM | 1.20 ± 0.89 | 20.9 ± 22.7 | 18.16 ± 13.33 | 18.08 ± 13.34 | 13.95 ± 11.23 | |
|
| ||||||
| 3,6-quinonec | BaP only | 0.48 ± 0.48 | 3.1 ± 3.3 | 4.45 ± 10.64 | 4.45 ± 10.64 | 3.61 ± 4.94 |
| Unknown Ec | DIM | 0.39 ± 0.49 | 16.8 ± 27.0 | 1.89 ± 4.62 | 1.88 ± 4.60 | 11.67 ± 18.71 |
|
| ||||||
| 9-OHc | BaP only | 1.49 ± 2.32 | 1.3 ± 0.6 | 2.17 ± 3.65 | 2.17 ± 3.65 | 1.22 ± 0.35 |
| BS | 0.17 ± 0.46 | 2.0 ± - | 0.13 ± 0.35 | 0.13 ± 0.35 | 1.39 ± - | |
|
| ||||||
| 3-OH | BaP only | 3.68 ± 3.38 | 1.5 ± 0.4 | 7.41 ± 7.51 | 7.41 ± 7.51 | 1.53 ± 0.64 |
| BS | 1.69 ± 1.02 | 1.2 ± 0.5 | 2.65 ± 1.79 | 2.65 ± 1.79 | 1.16 ± 0.53 | |
| DIM | 1.12 ± 2.17 | 1.3 ± 0.4 | 2.22 ± 5.19 | 2.22 ± 5.19 | 1.15 ± 0.49 | |
The values shown are the mean ± standard deviation of all participants
Toxicokinetic constants that were significantly different are shown in bold as determined by the Freidman test (Tmax only), or the likelihood ratio tests of mixed effect models.
3,6-quinone was not seen following either of the supplemented diets (Cmax was significantly different, p = 0.011), Unknown E was only seen following DIM supplementation (Cmax was significantly different, p = 0.037), and 9-OH was not seen following DIM supplementation.
Cmax Unknown A (p = 0.024) was significantly reduced following DIM supplementation; Brussels sprouts (9,10-dihydrodiol, p = 0.031; Unknown D, p = 0.030).
AUC(0,∞) of Unknown C was significantly reduced following both Brussels sprouts (p = 0.024) and DIM (p = 0.040) supplementation, as was Unknown A (full model p = 0.041), and Unknown D by Brussels sprouts (p = 0.037).
T1/2 was significantly reduced following Brussels sprouts (Unknown C, p = 0.037).
AUC was significantly reduced following Brussels sprouts (Unknown D, p = 0.019).
When examined individually, three participants (BaP022, BaP025 and BaP028) exhibited a marked reduction in the Cmax of 7,8-DHD (immediate precursor of BaP-7,8-DHD-9,10-E) plus the 7,8,9,10-tetraols (hydrolysis product of the 4 enantiomers of Bap-7,8-DHD-9,10-E) following a week of supplementation with either Brussels sprouts or DIM (Figure 7). One individual (BaP031, the lone female) showed increased formation of 7,8-DHD and tetraols following supplementation with DIM. The remaining three individuals showed little or no change. Individuals with the large decreases were the most robust producers of the precursor (7,8-DHD) and product (tetraols) of the ultimate BaP carcinogenic metabolite prior to supplementation.
Figure 7.
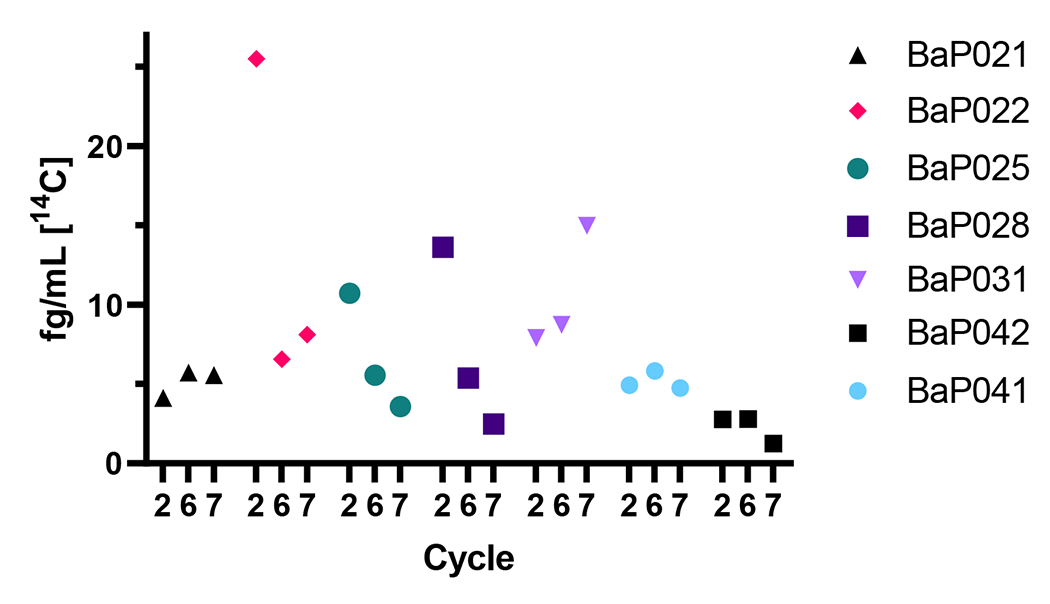
Impact of supplementation on metabolism of the bay region of [14C]-BaP. Sum of the [14C] representing Cmax (fg/mL plasma) for 7,8,9,10-tetraols and 7,8-dihydrodiol for each individual before (Cycle 2), or after one week of supplementation with Brussels sprouts (Cycle 6) or DIM (Cycle 7).
4. Discussion
The atto-zeptomole sensitivity of AMS allows for micro-dosing of humans with known [14C]-labeled carcinogens with de minimus risk (Enright et al., 2016; Hah et al., 2009). Initial micro-dosing studies with [14C]-chemical carcinogens examined covalent DNA damage utilizing a graphite technique that quantified total [14C] without speciation (Farmer et al., 2005). Development of a UPLC interface allows for identification (based on co-elution with standards) of metabolites along with the parent carcinogen (Thomas et al., 2011). Our group has used both approaches to examine the toxicokinetics of the PAHs DBC and BaP (Madeen et al., 2015; 2016; 2019; Hummel et al., 2018). The results with graphite (DBCeq and BaPeq) fit the PBPK model previously developed for rodents (Crowell et al., 2011; Pande et al., 2021) supporting its use in extrapolation to humans. The FDA (Kinders et al., 2007) has recognized the power of micro-dosing in Phase 0 protocols in part due to results such as from Lappin and Garner (2008) in which 15 of 18 drugs exhibited linear pharmacokinetics, when micro-dosed, not significantly different than therapeutic doses and consistent with our results to date with micro-dosing of PAHs. The potential value of AMS in cancer chemoprevention studies was discussed by Kensler and Groopman (2009) in conjuncture with the study by Jubert et al. (2009). As in the present study with BaP and DIM or Brussels sprouts, co-administration of chlorophyllin or purified chlorophylls to humans dosed with [14C]-aflatoxin B1 (AFB1) reduced the ka, Cmax and AUC (graphite analysis for total [14C]). The mechanism was postulated to be complexation (pi-pi stacking) of chlorophylls and CHL with AFB1 (observed in vitro and in preclinical models) inhibiting intestinal absorption. This mechanism is unlikely to apply in our study as all groups were fasted overnight prior to dosing with [14C]-BaP. In the DIM group, a final dose was given concurrent with [14C]-BaP administration, but there is no evidence of complexation of DIM with BaP. In a previous study to determine if coadministration of a complex mixture of PAHs would impact [14C]-BaP toxicokinetics, we fed participants 22 or 125 g of smoked salmon containing a complex mixture of PAHs (Confederated Tribes of the Umatilla Indian Reservation with 10-x the [14C]-BaP dose as BaPeq) or 180 g of commercially obtained canned salmon containing low levels of PAHs. The ka, Cmax, and AUC of [14C]-BaP were markedly reduced, but the effect was due to the quantity of salmon consumed and not the presence or absence of PAHs (Hummel et al., 2018). The impact of DIM or Brussels sprouts on kinetic constants could not be due to differences in stomach contents as all groups were fasted overnight and not given food until two h post [14C]-BaP dose.
Cruciferous vegetables and DIM have been thoroughly studied in preclinical models and have been the subject of numerous clinical trials (Ambrosone and Tang, 2009; Higdon et al., 2007; Kim and Park, 2009; Minich and Bland, 2007; van Poppel et al. 1999; Williams, 2021). In addition to I3C, the immediate precursor of DIM, cruciferous vegetables contain other cancer chemopreventive agents such as sulforaphane, fiber, and antioxidants supporting the assertion that the whole food would be superior to isolated bioactives such as I3C, DIM, and sulforaphane (Ambrosone and Tang, 2009; Higdon et al., 2007; Kim and Park, 2009; Minich and Bland, 2007; van Poppel et al. 1999; Williams, 2021). The overall impact of crucifer supplementation in human cancer prevention is positive, but not impressively so. A key factor is simply dose. Brussels sprouts are a rich source of glucobrassicin, the precursor of I3C and subsequently DIM, but one would need to consume over 10 kg (Shorey et al., 2013) to achieve the manufacturer’s daily-recommended dose of 300 mg (BR-DIM®, 90.6 mg DIM).
The mechanisms attributed to the chemopreventive properties of cruciferous vegetables, I3C, and DIM are numerous. One widely studied mechanism involves binding to and activating the aryl hydrocarbon receptor (AHR). Induction of phase 1 xenobiotic metabolizing enzymes such as cytochromes P450 (CYPs) and phase 2 enzymes such as glutathione-S-transferases (GSTs), UDP-glucuronosyl transferases (UGTs), and sulfotransferase (SULTs) would reduce metabolic activation of procarcinogens and enhance formation and excretion of non-toxic conjugates (Bjeldanes et al., 1991; Bradfield and Bjeldanes, 1987; Fahey and Kensler, 2007; Higdon et al., 2007; Lampe et al., 2000; Navarro et al., 2009; Peterson et al., 2009; Pool-Zobel, et al., 2005; Stresser et al., 1994; Williams, 2021; Wu et al., 2015). Enzyme induction is unlikely to explain the altered toxicokinetics in this study given that we did not see increased [14C]-BaP metabolites including conjugates.
The results from this study are consistent with our previous AMS studies with DBC and BaP in that a large interindividual variability is apparent. A previous study employing graphite AMS (total [14C] representing parent BaP plus metabolites) and repeated dosing of the same individual resulted in relatively small variations between dosings with no relationship to the length of the interval between dosing. In the present study, individuals (BaP022, 025 and 028) with the greatest reduction in total [14C] and bioactivation (bay region metabolites) were also those with the longest interval between dosings (201-237 days compared to 28-96 days). The intervals between DIM and Brussels sprout [14C]-BaP dosing were much shorter (28-48 days). If the interval length were important, this would be consistent with the similarity in BaP toxicokinetics in those groups.
Inter-individual variability of BaP metabolism is a function of genetics, environment, and gene-environment interactions. Our study design attempted to minimize the environmental impact with dietary restrictions, food diaries, and rigorous quality control in the preparation and administration of the [14C]-BaP dose as well as sample collection, extraction, and analysis. Based on the dietary questionnaire, the mean daily BaP consumption during the three pre-dosing cycles exhibited low estimates of 189 ± 78, 201 ± 52, and 187 ± 114 ng/day and highs of 240 ± 76, 283 ± 82, and 262 ± 170 ng/day which fall in the low range of the JEFCA (2005) estimate (250-700 ng/day) for non-smokers in the US. During the 48 h of the three studies the mean for the low estimates of daily dietary BaP were 454 ± 275, 253 ± 134, and 201 ± 83 with high estimates of 571 ± 369, 324 ± 155, and 273 ± 113 ng/day. The enzymes involved in phase 1 and phase 2 metabolism of BaP, as well as transporters, exhibit genetic polymorphisms and would be expected to be a large contributor to intraindividual variability in BaP toxicokinetics. The participant with the greatest production of 7,8-DHD and 7,8,9,10-tetraols (BaP022) is homozygous for the CYP1B1*3 and GSTM1*0 (null) alleles and a CYP1A2 genotype (163C>A; 5347C>T) (data not shown) associated with ultrarapid metabolism, all of which would be consistent with enhanced production of these bay region metabolites.
Previous work has shown that urinary levels of DIM are reliable biomarkers for the consumption of Brussels sprouts despite reported issues of participant compliance due to the high volume of raw Brussels sprouts required (Fujioka et al., 2016). In an effort to have presumed urine levels of DIM be within the range of those shown to be reliable biomarkers and encourage volunteer compliance, 50 g Brussels sprouts were consumed, thawed, warmed, and seasoned as desired. Glucosinolates and subsequently glucobrassicin concentrations in crucifers vary by cultivar, and are effected by harvesting, storage, and cooking methods, potentially altering downstream concentrations of I3C and DIM (McNaughton and Marks, 2007). Brussel sprout glucobrassicin levels were not quantified in this study, however, in order to control for variation in the Brussels sprouts between participants all provided Brussels sprouts purchased were the same brand and lot number, then combined, weighed, and stored together in individual vacuum pouches. Following the Brussels sprout regimen in this study, DIM and DIM metabolites were not quantifiable in participants fasted overnight, consistent with previous work (Fujioka et al., 2016).
The final dose of DIM was administered to fasted participants just prior to administration of [14C]-BaP. We adopted this strategy to ensure we would be able to observe effects due to direct inhibition as well as enzyme induction. Our recent study found a T1/2 in plasma of 4.3 ± 2.5 h in agreement with pharmacokinetic studies of DIM in humans (Maier et al., 2021a; Reed et al., 2008). Although parent DIM was not detected in participants fasted overnight, substantial amounts of the sulfate and glucuronide conjugates were present (Maier et al., 2021b). These results confirm that little or no DIM would be present in plasma the morning of [14C]-BaP dosing. Our laboratory and others have shown DIM to be a non-specific inhibitor of multiple rodent and human CYPs with Kis in the low μM range (Parkin et al., 2008; Schiering et al., 2017; Stresser et al., 1995; Williams, 2021). If the liver/plasma ratio is similar to mice (Anderton et al., 2004a; 2004b), based on the Cmax we observed (~0.4 μM), estimated liver concentrations of DIM in this study would be 3-4 μM and in the range expected to inhibit human CYPs 1A1, 1A2, and 3A4 (Williams, 2021). In addition, we would expect that if CYP inhibition were responsible for the alteration of toxicokinetics, there would be a corresponding increase in unmetabolized BaP. The yield of bay region metabolites (bioactivation) remained constant (32-34%) across all groups, again arguing against DIM inhibition of CYP-dependent BaP metabolism as the mechanism involved as is the observation that the Cmax for metabolites follow the same pattern as for parent BaP Cmax while Clint is not altered. The markedly reduced Tmax and Cmax for BaP and reduced Cmax of four downstream BaP metabolites for [14C]-BaP are indicative of slower absorption. Overall, both Brussels sprouts and more so Dim appear to reduce the overall absorption of BaP, however the variability between participants, and subsequent pharmacokinetic parameters, does not allow for adequate statistical evidence. DIM is known to enhance intestinal barrier integrity and alter xenobiotic transporters which could explain the reduction in overall [14C] with no impact on metabolite profiles (Kim et al., 2019; Metidji et al., 2018; Penta et al., 2021; Schiering et al., 2017). The issue with any of the mechanisms for DIM discussed above is that it does not account for the similar effects observed following one week of dietary supplementation with Brussels sprouts.
This study demonstrated that a whole food and dietary supplement, described as effective dietary chemopreventives in numerous preclinical cancer models, showed promise in this small human clinical trial. Validation of AMS micro-dosing studies for establishing absorption, metabolism, distribution and excretion (ADME) in drug development (CREAM study, Lappin et al., 2006) prompted the FDA to approve a Phase 0 approach saving pharmaceutical companies time and money needed to conduct a typical Phase 1 trial (FDA, 2008). In drug development, phase 0 trials can also be used for establishing a lead candidate (Kinders et al., 2007). Kensler and Groopman (2009), in proposing the use of AMS in chemoprevention studies, suggested the term “reverse phase 0” for human trials involving micro-dosing with carcinogens to evaluate chemopreventive agents. The atto- to zepto-mole sensitivity allows analysis of chemopreventive agent-dependent alterations in carcinogen toxicokinetics in vivo in humans (as was done in this study). The exquisite sensitivity of UPLC-AMS also allows for detection of carcinogen-DNA adducts (and their identity) with a sensitivity (1 adduct in 1011-1012 bp) 10,000-fold greater than conventional LC-MS/MS adductions (Brown et al., 2006). Comparison of DNA adduction (an important tool in cancer chemoprevention studies) following treatment with MeIQx to humans and rodents in vivo showed a 10-x greater level of adduction per unit dose in humans (Turteltaub et al., 1997; 1999) highlighting the perspective as discussed by Seymour (2009) that “The Best Model for Humans is Humans”. The current cost/sample is about $500 for UPLC-AMS. The low number (4-8) of participants needed (Penner et al., 2012) and use of a single dose followed over 2-3 days (rather than multiple dosing over months) results in a cost of about $9,000 per participant for a complete time-course of plasma and urine analysis. A 250 kV AMS is currently 2-3-times the cost of a conventional triple quadrupole mass spectrometer and the cost and availability of AMS instrumentation continues to decrease. For all of these reasons, the use of UPLC-AMS in “reverse phase 0” human clinical trials has a great deal of potential utility and should be an important tool in cancer chemoprevention. Although this study could not definitely establish a mechanism of chemoprevention for DIM or Brussels sprouts, we were able to eliminate alteration of metabolism of [14C]-BaP. Future studies could examine potential mechanisms related to absorption and DIM or Brussels sprout impact on the microbiome.
5. Conclusions
This study employed UPLC-AMS to assess the potential for the whole food Brussels sprouts or DIM, a dietary supplement that is a major bioactive and known cancer chemopreventive agent in preclinical models, to alter the toxicokinetics of a known human dietary carcinogen. Although not uniformly statistically significant, due to a large interindividual variability among the seven subjects, one week of supplementation with either Brussels sprouts or DIM did significantly reduce the Cmax for parent BaP as well as four of the eleven metabolites observed. Dietary treatments also appeared to reduce the T1/2 and the plasma AUC(0,∞) for Unknown Metabolite C, indicating some effect in accelerating clearance of this metabolite. This reduction occurred with little change in the overall metabolite profile and no change in the relative oxygenation at the bay region of BaP. Our toxicokinetic analysis suggest DIM and Brussels sprout supplementation reduced the rate but not the extent of BaP absorption from the gut. Although DIM is a known inhibitor of CYPs, the observation that the Cmax for metabolites followed the same pattern as BaP and overall Cl rates for these metabolites was not significantly impacted, argues against DIM inhibition of CYP-dependent BaP metabolism as the mechanism. This is the first study in humans documenting the impact of a whole food or dietary supplement on the toxicokinetics, including metabolic profiles, of a known dose of an IARC group 1 human carcinogen.
Supplementary Material
Highlights.
[14C]-Benzo[a]pyrene (BaP) toxicokinetics (TK) were studied in humans with UPLC-AMS
The rate but not extent of BaP absorption was reduced by Brussels sprouts and DIM
Intervention reduced total [14C] recovered from plasma by 56-67%
TK constants such as clearance and the metabolic profile of BaP were unchanged
Impacts on TK are due to gut absorption of BaP, not alteration of metabolism
Acknowledgements
The authors would like to thank the participants who volunteered to be part of this study. Special thanks are also given to Carolyn Kinzie, Danny Chen, and Peter Scruggs for their contributions with sample extractions and preparation.
Funding sources
This study was funded by Public Health Service NIH grants P42ES016465, R01ES028600, T32ES007060, and P30ES030287. Work performed in part at the National User Resource for Biological Accelerator Mass Spectrometry, which is operated at LLNL under the auspices of the US Department of Energy under contract DE-AC52-07NA27344. The User Resource is supported by the National Institutes of Health (NIH), National Institute of General Medical Sciences (NIGMS) under grant R24GM137748.
Abbreviations
- AFB1
aflatoxin B1
- AMS
accelerator mass spectrometry
- AHR
aryl hydrocarbon receptor
- AUC
area under the curve
- ATSDR
Agency for Toxic Substances and Disease Registry
- BaP
benzo[a]pyrene
- BaP-7,8-DHD
(±)-trans-benzo[a]pyrene-dihydrodiol
- BaP-DHDE
BaP-7,8-DHD-9,10-epoxide
- BMI
body mass index
- BR-DIM
BioResponse 3,3’diindolylmethane
- Cmax
maximum concentration in plasma
- CHL
chlorophyllin
- CYP
cytochrome P-450
- DBC
dibenzo[def,p]chrysene
- DIM
3,3’-diindolylmethane
- FDA IND
US Food and Drug Administration Investigational New Drug
- HCC
hepatocellular carcinoma
- IARC
International Agency for Research on Cancer
- IPCS
International Programme on Chemical Safety
- IQ
2-amino-3-methylimidazo[4,5-f]quinolone
- IRB
Institutional Review Board
- JEFCA
Joint FAO/WHO Expert Committee on Food Additives
- LLNL
Lawrence Livermore National Laboratory
- LOD
limit of detection
- LOQ
limit of quantitation
- MeIQx
2-amino-3,8-dimethylimidazo[4,5-b]quinoxaline
- NPL
National Priorities List
- N2-dG
nitrogen at the 2 position of deoxyguanosine
- OSU
Oregon State University
- PAH
polycyclic aromatic hydrocarbon
- PBMC
peripheral blood mononucleated cell
- PBPK
physiologically based pharmacokinetics
- PhIP
2-amino-3-methyl-6-phenylimidazo[4,5-b]pyridine
- RPF
Relative Potency Factor
- Tmax
time at Cmax
- UPLC
ultra-pressure liquid chromatography (Waters)
- US EPA
United States Environmental Protection Agency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT authorship contribution statement
Monica L. Vermillion Maier: Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing-original draft; Writing-review and editing. Lisbeth K. Siddens: Data curation; Investigation; Methodology; Supervision; Validation; Writing-review and editing. Jamie M. Pennington: Data curation; Investigation; Writing-review and editing. Sandra L. Uesugi: Data curation; Methodology; Project administration, Resources; Supervision; Writing-review and editing. Susan C. Tilton: Methodology; Resources; Supervision; Writing-review and editing. Emily A. Vertel: Data curation. Kim A. Anderson: Data curation; Formal analysis; Methodology; Resources; Validation. Lane G. Tidwell: Formal analysis; Writing-review and editing. Ted J. Ognibene: Formal analysis; Methodology; Resources; Software; Validation; Writing-review and editing. Kenneth W. Turteltaub: Project administration, Resources. Jordan N. Smith: Formal analysis; Methodology; Software; Visualization; Writing-original draft; Writing-review and editing. David E. Williams: Conceptualization; Funding acquisition; Investigation; Project administration, Resources; Supervision; Validation; Writing-original draft; Writing-review and editing.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
5. References
- Ambrosone CB, Tang L (2009) Cruciferous vegetable intake and cancer prevention: role of nutrigenetics. Cancer Prev. Res 2, 298–300, doi: 10.1158/1940-6207.CAPR-09-0037. [DOI] [PubMed] [Google Scholar]
- Anderton MJ, Manson MM, Verschoyle RD, Gescher A, Lamb JH, Farmer PB, Steward WP, Williams ML (2004a) Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice, Clin. Cancer Res 10, 5233–41, doi: 10.1158/1078-0432.CCR-04-0163. [DOI] [PubMed] [Google Scholar]
- Anderton MJ, Manson MM, Verschoyle R, Gescher A, Steward WP, Williams ML, Mager DE (2004b) Physiological modeling of formulated and crystalline 3,3’-diindolylmethane pharmacokinetics following oral administration in mice. Drug Metabol. Disp 32, 632–8, doi: 10.1124/dmd.32.6.632. [DOI] [PubMed] [Google Scholar]
- ATSDR (1995) Agency for Toxic Substances and Disease Registry. Toxicological Profile for Polycyclic Aromatic Hydrocarbons; pp. 1–487. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=122&tid=25, (accessed June 15, 2022). [PubMed] [Google Scholar]
- ATSDR (2019) https://www.atsdr.cdc.gov/spl/index.html, (accessed June 15, 2022).
- Bansal V, Kim K-H (2015) Review of PAH contamination in food products and their health hazards. Environ. Int 84, 26–38, 10.1016/j.envint.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J. Stat. Software 67, 1–48. Doi, 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Bjeldanes LF, Kim J-Y, Grose KR, Bartholomew JC, Bradfield CA (1991) Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc. Natl. Acad. Sci. (USA) 88, 9543–7, doi: 10.1073/pnas.88.21.9543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfield CA, Bjeldanes LF (1987) Structure-activity relationships of dietary indoles: a proposed mechanism of action as modifiers of xenobiotic metabolism. J. Toxicol. Environ. Health 21, 311–23, doi: 10.1080/15287398709531021. [DOI] [PubMed] [Google Scholar]
- Brown K, Tompkins EM, White IN (2006) Applications of accelerator mass spectrometry for pharmacological and toxicological research. Mass Spectrom. Rev 25, 127–45, doi: 10.1002/mas.20059. [DOI] [PubMed] [Google Scholar]
- Conney AH, Chang RL, Jerina DM, Wei S-JC (1994) Studies on the metabolism of benzo[a]pyrene and dose-dependent differences in the mutagenic profile of Its ultimate carcinogenic metabolite. Drug Metab. Rev 26, 125–63, 10.3109/03602539409029788. [DOI] [PubMed] [Google Scholar]
- Crowell SR, Amin SG, Anderson KA, Krishnegowda G, Sharma AK, Soelberg JJ, Williams DE, Corley RA (2011) Preliminary physiologically based pharmacokinetic models for benzo[a]pyrene and dibenzo[def,p]chrysene in rodents. Toxicol. Appl. Pharmacol 257, 365–76, doi: 10.1016/j.taap.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell SR, Hanson-Drury S, Williams DE, Corley RA (2014) In vitro metabolism of benzo[a]pyrene and dibenzo[def,p]chrysene in rodent and human hepatic microsomes. Toxicol. Lett 228, 48–55, doi: 10.1016/j.toxlet.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupid BC, Lightfoot TJ, Russell D, Gant SJ, Turner PC, Dingley KH, Curtis KD, Leveson SH, Turteltaub KW, Garner RC (2004) The formation of AFB(1)-macromolecular adducts in rats and humans at dietary levels of exposure. Fd. Chem. Toxicol 42, 559–69, doi: 10.1016/j.fct.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Nadal M (2015) Human dietary exposure to polycyclic aromatic hydrocarbons: A review of the scientific literature. Fd. Chem. Toxicol 86, 144–53, 10.1016/j.fct.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Dueker SR, Vuong LT, Lohstroh PN, Giacomo JA, Vogel JS (2011) Quantifying exploratory low dose compounds in humans with AMS. Adv. Drug Deliv. Rev 63, 518–31, doi: 10.1016/j.addr.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority (EFSA) (2008) Scientific opinion of the panel on contaminants in the food chain on a request from the European Commission on Polycyclic Aromatic Hydrocarbons in Food. EFSA J. 724, 1–114. [Google Scholar]
- Egner PA, Wang JB, Zhu YR, Zhang BC, Wu Y, Zhang QN, Qian GS, Kuang SY, Gange SJ, Jacobson LP, Helzlsouer KJ, Bailey GS, Groopman JD, Kensler TW (2001) Chlorophyllin intervention reduces aflatoxin-DNA adducts in individuals at high risk for liver cancer. Proc. Natl. Acad. Sci. (USA) 98, 14601–6, doi: 10.1073/pnas.251536898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright HA, Malfatti MA, Zimmermann M, Ognibene T, Henderson P, Turteltaub KW (2016) Use of accelerator mass spectrometry in human health and molecular toxicology. Chem. Res. Toxicol 29, 1976–86, doi: 10.1021/acs.chemrestox.6b00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey JW, Kensler TW (2007) Role of dietary supplements/nutraceuticals in chemoprevention through induction of cytoprotective enzymes. Chem. Res. Toxicol 20, 572–6, doi: 10.1021/tx7000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer PB, Brown K, Tompkins E, Emms VL, Jones DJL, Singh R, Phillips DH (2005) DNA adducts: mass spectrometry methods and future prospects. Toxicol. Appl. Pharmacol 207(2 Suppl), 293–301, doi: 10.1016/j.taap.2004.12.020. [DOI] [PubMed] [Google Scholar]
- FDA (2008). Guidance for Industry: Safety Testing of Drug Metabolites. Rockville, MD, USA: US Department of Health & Human Services, Food & Drug Administration, Center for Drug Evaluation and Research (CDER). [Google Scholar]
- Fujioka N, Ransom BW, Carmella SG, Upadhyaya P, Lindgren BR, Roper-Batker A, Hatsukami DK, Fritz VA, Rohwer C, Hecht SS (2016) Harnessing the power of cruciferous vegetables: Developing a biomarker for Brassica vegetable consumption using urinary 3,3’-diindolylmethane. Cancer Prev. Res 9, 788–93, doi: 10.1158/1940-6207.CAPR-16-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier MC,Minick DJ, Truong L, Tilton S, Pande P, Anderson KA, Teeguardan J, Tanguay RL (2018) Systematic developmental neurotoxicity assessment of a representative PAH Superfund mixture using zebrafish. Toxicol. Appl. Pharmacol 354, 115–25, doi: 10.1016/j.taap.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibaldi M and Perrier D, 1982. Pharmacokinetics, M. Dekker, New York. [Google Scholar]
- Hah SS, Henderson PT, Turteltaub KW (2009) Recent advances in biomedical applications of accelerator mass spectrometry. J. Biomed. Sci 16, 54, doi: 10.1186/1423-0127-16-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, Kelleher MO, Eggleston IM (2008) The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur. J. Nutr 47(Suppl 2), 73–88, doi 10.1007/s00394-008-2009-8. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Delage B, Williams DE, Dashwood RH (2007) Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res 55, 224–36, doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Mesaros C, Hackfeld LC, Hodge RP, Zang T, Blair IA, Penning TM (2017) Potential metabolic activation of a representative C4-alkylated polycyclic aromatic hydrocarbon retene (1-methyl-7-isopropyl-phenanthrene) associated with the Deepwater Horizon oil spill in human hepatoma (HepG2) cells. Chem. Res. Toxicol 30, 1093–1101, doi: 10.1021/acs.chemrestox.6b00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel JM, Madeen EP, Siddens LK, Uesugi SL, McQuistan T, Anderson KA, Turteltaub KW, Ognibene TJ, Bench G, Krueger SK, Harris S, Smith J, Tilton SC, Baird WM, Williams DE (2018) Pharmacokinetics of [14C]-benzo[a]pyrene (BaP) in humans: Impact of co-administration of smoked salmon and BaP dietary restriction. Fd. Chem. Toxicol 115, 136–47, 10.1016/j.fct.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (1983) IARC Working Group on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Polynuclear aromatic compounds, Part 1: Chemical, environmental and experimental data. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; International Agency for Research on Cancer; 32, 1–453, https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Polynuclear-Aromatic-Compounds-Part-1-Chemical-Environmental-And-Experimental-Data-1983, (accessed June 16, 2022). [PubMed] [Google Scholar]
- IARC (2004) IARC Handbooks of Cancer Prevention, Cruciferous Vegetables, Isothiocyanates and Indoles. International Agency for Research on Cancer; 9, 1–265, https://publications.iarc.fr/Book-And-Report-Series/Iarc-Handbooks-Of-Cancer-Prevention/Cruciferous-Vegetables-Isothiocyanates-And-Indoles-2004 (accessed June 14, 2022). [Google Scholar]
- IARC (2010) IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer; 92, https://www.ncbi.nlm.nih.gov/books/NBK321712/, (accessed June 16, 2022). [PMC free article] [PubMed] [Google Scholar]
- Jakszyn P, Agudo A, Ibáñez R, García-Closas R, Pera G, Amiano P, González CA (2004) Development of a food database of nitrosamines, heterocyclic amines, and polycyclic aromatic hydrocarbons. J. Nutr 134, 2011–4, doi: 10.1093/jn/134.8.2011. [DOI] [PubMed] [Google Scholar]
- JEFCA (Joint FAO/WHO Expert Committee on Food Additives) (2005) Sixty-fourth Meeting (JECFA/64/SC), Rome, https://www.fao.org/documents/card/en/c/f95956a6-c4ac-4843-bd48-b6a1fbe3afac/ (accessed June 16, 2022). [Google Scholar]
- Jubert C, Mata J, Bench G, Dashwood R, Pereira C, Tracewell W, Turteltaub K, Williams D, Bailey G (2009) Effects of chlorophyll and chlorophyllin on low-dose aflatoxin B(1) pharmacokinetics in human volunteers. Cancer Prev. Res 2, 1015–22, doi: 10.1158/1940-6207.CAPR-09-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitulnik J, Wislocki PG, Levin W, Yagi H, Jerina DM, Conney AH (1978) Tumorigenicity studies with diol-epoxides of benzo(a)pyrene which indicate that (+/−)-trans-7beta,8alpha-dihydroxy-9alpha, 10alpha-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene is an ultimate carcinogen in newborn mice. Cancer Res. 38, 354–8. [PubMed] [Google Scholar]
- Kazerouni N, Sinha R, Hsu CH, Greenberg A, Rothman N (2001) Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Fd. Chem. Toxicol 39, 423–36, 10.1016/s0278-6915(00)00158-7. [DOI] [PubMed] [Google Scholar]
- Keck BD, Ognibene T, Vogel JS (2010) Analytical validation of accelerator mass spectrometry for pharmaceutical development. Bioanalysis 2, 469–85, doi: 10.4155/bio.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Groopman JD (2009) Is it time to advance the chemoprevention of environmental carcinogenesis with microdosing trials? Cancer Prev. Res 2, 1003–7, doi: 10.1158/1940-6207.CAPR-09-0232. [DOI] [PubMed] [Google Scholar]
- Kim MK, Park JHY (2009) Conference on “Multidisciplinary approaches to nutritional problems”. Symposium on “Nutrition and health”. Cruciferous vegetable intake and the risk of human cancer: epidemiological evidence. Proc. Nutr. Soc 68, 103–10, doi: 10.1017/S0029665108008884. [DOI] [PubMed] [Google Scholar]
- Kim JY, Le TAN, Lee SY, Song DG, Hong SC, Cha KH, Lee JW, Pan CH, Kang K (2019) 3,3’-Diindolylmethane improves intestinal permeability dysfunction in cultured human intestinal cells and the model animal Caenorhabditis elegans. J. Agric. Fd. Chem 67, 9277–85, doi: 10.1021/acs.jafc.9b03039. [DOI] [PubMed] [Google Scholar]
- Kinders R, Parchment RE, Ji J, Kummar S, Murgo AJ, Gutierrez M, Collins J, Rubinstein L, Pickeral O, Steinberg SM, Yang S, Hollingshead M, Chen A, Helman L, Wiltrout R, Simpson M, Tomaszewski JE, Doroshow JH (2007) Phase 0 clinical trials in cancer drug development: from FDA guidance to clinical practice. Mol. Interv 7, 325–34, doi: 10.1124/mi.7.6.9. [DOI] [PubMed] [Google Scholar]
- Lampe JW, Chen C, Li S, Prunty J, Grate MT, Meehan DE, Barale KV, Dightman DA, Feng Z, Potter JD (2000) Modulation of human glutathione S-transferases by botanically defined vegetable diets. Cancer Epidemiol. Biomarkers Prev 9, 787–93. [PubMed] [Google Scholar]
- Lappin G, Garner RC (2008) The utility of microdosing over the past 5 years. Expert Opin. Drug Metab. Toxicol. 4, 1499–506, doi: 10.1517/17425250802531767. [DOI] [PubMed] [Google Scholar]
- Lappin G, Kuhnz W, Jochemsen R, Kneer J, Chaudhary A, Oosterhuis B, Drijfhout WJ, Rowland M, Garner RC (2006) Use of microdosing to predict pharmacokinetics at the therapeutic dose: experience with 5 drugs. Clin. Pharmacol. Ther 80, 203–15, doi: 10.1016/j.clpt.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Lodovici M, Luceri C, Guglielmi F, Bacci C, Akpan V, Fonnesu ML, Boddi V, Dolara P (2004) Benzo(a)pyrene diolepoxide (BPDE)-DNA adduct levels in leukocytes of smokers in relation to polymorphism of CYP1A1, GSTM1, GSTP1, GSTT1, and mEH. Cancer Epidemiol. Biomarkers Prev 13, 1342–8. [PubMed] [Google Scholar]
- Madeen E, Corley RA, Crowell S, Turteltaub K, Ognibene T, Malfatti M, McQuistan TJ, Garrard M, Sudakin D, Williams DE (2015) Human in vivo pharmacokinetics of [(14)C]dibenzo[def,p]chrysene by accelerator mass spectrometry following oral microdosing. Chem. Res. Toxicol 28, 126–34, 10.1021/tx5003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeen EP, Ognibene TJ, Corley RA, McQuistan TJ, Henderson MC, Baird WM, Bench G, Turteltaub KW, Williams DE (2016) Human microdosing with carcinogenic polycyclic aromatic hydrocarbons: In vivo pharmacokinetics of dibenzo[def,p]chrysene and metabolites by UPLC accelerator mass spectrometry. Chem. Res. Toxicol 29, 1641–50, 10.1021/acs.chemrestox.6b00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeen E, Siddens LK, Uesugi S, McQuistan T, Corley RA, Smith J, Waters KM, Tilton SC, Anderson KA, Ognibene T, Turteltaub K, Williams DE (2019) Toxicokinetics of benzo[a]pyrene in humans: Extensive metabolism as determined by UPLC-accelerator mass spectrometry following oral micro-dosing. Toxicol. Appl. Pharmacol 364, 97–105, 10.1016/j.taap.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier MLV, Siddens LK, Pennington JM, Uesugi SL, Anderson KA, Tidwell LG, Tilton SC, Ognibene TJ, Turteltaub KW, Smith JN, Williams DE (2021a) Benzo[a]pyrene (BaP) metabolites predominant in human plasma following escalating oral micro-dosing with [14C]-BaP. Environ. Int 159,107045, doi: 10.1016/j.envint.2021.107045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier MLV, Siddens LK, Uesugi SL, Choi J, Leonard SW, Pennington JM, Tilton SC, Smith JN, Ho E, Chow HHS, Nguyen BD, Kolluri SK, Williams DE (2021b) 3,3’-Diindolylmethane exhibits significant metabolism after oral dosing in humans. Drug Metabol. Dispos 49, 694–705, doi: 10.1124/dmd.120.000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfatti MA, Kulp KS, Knize MG, Davis C, Massengill JP, Williams S, Nowell S, MacLeod S, Dingley KH, Turteltaub KW, Lang NP, Felton JS (1999) The identification of [2-14C]-2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine metabolites in humans. Carcinogenesis 20, 705–13, doi: 10.1093/carcin/20.4.705. [DOI] [PubMed] [Google Scholar]
- McNaughton S, & Marks G (2003). Development of a food composition database for the estimation of dietary intakes of glucosinolates, the biologically active constituents of cruciferous vegetables. British Journal of Nutrition, 90(3)687–697. doi: 10.1079/BJN2003917 [DOI] [PubMed] [Google Scholar]
- Mensing T, Marczynski B, Engelhardt B, Wilhelm M, Preuss R, Kappler M, Angerer J, Kafferlein HU, Scherenberg M, Seidel A, Brüning T (2005) DNA adduct formation of benzo[a]pyrene in white blood cells of workers exposed to polycyclic aromatic hydrocarbons. Int. J. Hyg. Environ. Health 208, 173–8, doi: 10.1016/j.ijheh.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Metidji A, Omenetti S, Crotta S, Li Y, Nye E, Ross E, Li V, Maradana MR, Schiering C, Stockinger B (2018) The environmental sensor AHR protects from inflammatory damage by maintaining intestinal stem cell homeostasis and barrier integrity. Immunity 49, 353–62, doi: 10.1016/j.immuni.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minich DM, Bland JS (2007) A review of the clinical efficacy and safety of cruciferous vegetable phytochemicals. Nutr. Rev 65, 259–67, doi: 10.1301/nr.2007.jun.259-267. [DOI] [PubMed] [Google Scholar]
- Navarro SL, Peterson S, Chen C, Makar KW, Schwarz Y, King IB, Li SS, Li L, Kestin M, Lampe JW (2009) Cruciferous vegetable feeding alters UGT1A1 activity: diet- and genotype-dependent changes in serum bilirubin in a controlled feeding trial. Cancer Prev. Res 2, 345–52, doi: 10.1158/1940-6207.CAPR-08-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal MS, Zhu J, Foster WG (2008) Quantification of benzo[a]pyrene and other PAHs in the serum and follicular fluid of smokers versus non-smokers. Reprod. Toxicol 25, 100–6, doi: 10.1016/j.reprotox.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Ognibene TJ, Thomas AT, Daley PF, Bench G, Turteltaub KW (2015) An interface for the direct coupling of small liquid samples to AMS. Nucl. Instrum. Methods Phys. Res. B 361, 173–7, doi: 10.1016/j.nimb.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ognibene TJ, Haack KW, Bench G, Turteltaub KW (2019) Trials and tribulations in the first three years in operation of the SSAMS for biomedical 14C-AMS at LLNL. Nucl. Instrum. Methods Phys. Res. B 438, 166–71, doi: 10.1016/j.nimb.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne MR, Beland FA, Harvey RG, Brookes P (1976) The reaction of (+/−)-7alpha, 8beta-dihydroxy-9beta, 10beta-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene with DNA. Int. J. Cancer 18, 362–8, doi: 10.1002/ijc.2910180315. [DOI] [PubMed] [Google Scholar]
- Pande P, Madeen EP, Williams DE, Crowell SR, Ognibene TJ, Turteltaub KW, Corley RA, Smith JN (2021) Translating dosimetry of dibenzo[def,p]chrysene (DBC) and metabolites across dose and species using physiologically based pharmacokinetic (PBPK) modeling. Toxicol. Appl. Pharmacol 438, 115830, doi: 10.1016/j.taap.2021.115830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DR, Lu Y, Bliss RL, Malejka-Giganti D (2008) Inhibitory effects of a dietary phytochemical 3,3’-diindolylmethane on the phenobarbital-induced hepatic CYP mRNA expression and CYP-catalyzed reactions in female rats. Fd. Chem. Toxicol 46, 2451–8, doi: 10.1016/j.fct.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Penner N, Xu L, Prakash C (2012) Radiolabeled absorption, distribution, metabolism, and excretion studies in drug development: why, when, and how? Chem. Res. Toxicol 25, 513–31, doi: 10.1021/tx300050f. [DOI] [PubMed] [Google Scholar]
- Penta D, Mondal P, Natesh J, Meeran SM (2021) Dietary bioactive diindolylmethane enhances the therapeutic efficacy of centchroman in breast cancer cells by regulating ABCB1/P-gp efflux transporter. J. Nutr. Biochem 94, 108749, doi: 10.1016/j.jnutbio.2021.108749. [DOI] [PubMed] [Google Scholar]
- Perera F, Tang D, Whyatt R, Lederman SA, Jedrychowski W (2005) DNA damage from polycyclic aromatic hydrocarbons measured by benzo[a]pyrene-DNA adducts in mothers and newborns from Northern Manhattan, the World Trade Center Area, Poland, and China. Cancer Epidemiol. Biomarkers Prev 14, 709–14, doi: 10.1158/1055-9965.EPI-04-0457. [DOI] [PubMed] [Google Scholar]
- Peterson S, Schwarz Y, Li SS, Li L, King IB, Chen C, Eaton DL, Potter JD, Lampe JW (2009) CYP1A2, GSTM1, and GSTT1 polymorphisms and diet effects on CYP1A2 activity in a crossover feeding trial. Cancer Epidemiol. Biomarkers Prev 18, 3118–25, doi: 10.1158/1055-9965.EPI-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil JD, Stiegel MA, Sobus JR, Tabucchi S, Ghio AJ, Madden MC (2010) Cumulative exposure assessment for trace-level polycyclic aromatic hydrocarbons (PAHs) using human blood and plasma analysis. J. Chromatogr. B 878, 1753–60, 10.1016/j.jchromb.2010.04.035. [DOI] [PubMed] [Google Scholar]
- Pool-Zobel B, Veeriah S, Böhmer FD (2005) Modulation of xenobiotic metabolising enzymes by anticarcinogens -- focus on glutathione S-transferases and their role as targets of dietary chemoprevention in colorectal carcinogenesis. Mutat. Res 591(1-2), 74–92, doi: 10.1016/j.mrfmmm.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Reed GA, Sunega JM, Sullivan DK, Gray JC, Mayo MS, Crowell JA, Hurwitz A (2008) Single-dose pharmacokinetics and tolerability of absorption enhanced 3,3’-diindolylmethane in healthy subjects. Cancer Epidemol. Biomarkers Prev 17, 2619–24, doi: 10.1158/1055-9965.EPI-08-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehpour M, Possnert G, Bryhni H (2008) Subattomole sensitivity in biological accelerator mass spectrometry. Anal. Chem 80, 3515–21, 10.1021/ac800174j. [DOI] [PubMed] [Google Scholar]
- Sampaio GR, Guizellini GM, da Silva SA, de Almeida AP, Pinaffi-Langley ACC, Rogero MM, de Camargo AC, Torres EAFS (2021) Polycyclic aromatic hydrocarbons in foods: Biological effects, legislation, occurrence, analytical methods, and strategies to reduce their formation. Int. J. Mol. Sci 22, 6010, doi: 10.3390/ijms22116010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiering C, Wincent E, Metidji A, Iseppon A, Li Y, Potocnik AJ, Omenetti S, Henderson CJ, Wolf CR, Nebert DW, Stockinger B (2017) Feedback control of AHR signaling regulates intestinal immunity. Nature 542, 242–5, doi: 10.1038/nature21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepkovic DW, Bradlow HL, Bell M (2001) Quantitative determination of 3,3’-diindolylmethane in urine of individuals receiving indole-3-carbinol. Nutr. Cancer 41, 57–63, doi: 10.1080/01635581.2001.9680612. [DOI] [PubMed] [Google Scholar]
- Seymour M. (2009) The best model for humans is human – how to accelerate early drug development safely. Altern. Lab. Anim 37 Suppl 1, 61–5, doi: 10.1177/026119290903701S09. [DOI] [PubMed] [Google Scholar]
- Shimada T. (2006) Xenobiotic-metabolizing enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug Metabol. Pharmacokinet 21, 257–76, doi: 10.2133/dmpk.21.257. [DOI] [PubMed] [Google Scholar]
- Shimada T, Fujii-Kuriyama Y (2004) Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 95, 1–6, 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorey LE, Madeen EP, Atwell LL, Ho E, Löhr CV, Pereira CB, Dashwood RH, Williams DE (2013) Differential modulation of dibenzo[def,p]chrysene transplacental carcinogenesis: Maternal diets rich in indole-3-carbinol versus sulforaphane. Toxicol. Appl. Pharmacol 270, 60–9, 10.1016/j.taap.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stresser DM, Bjeldanes LF, Bailey GS, Williams DE (1995) The anticarcinogen 3,3’-diindolylmethane is an inhibitor of cytochrome P-450. J. Biochem. Toxicol 10, 191–201, doi: 10.1002/jbt.2570100403. [DOI] [PubMed] [Google Scholar]
- Stresser DM, Williams DE, McLellan LI, Harris TM, Bailey GS (1994) Indole-3-carbinol induces a rat liver glutathione transferase subunit (Yc2) with high activity toward aflatoxin B1 exo-epoxide. Association with reduced levels of hepatic aflatoxin-DNA adducts in vivo. Drug Metab. Dispos 22, 392–9. [PubMed] [Google Scholar]
- Thomas AT, Ognibene T, Daley P, Turteltaub K, Radousky H, Bench G (2011) Ultrahigh efficiency moving wire combustion interface for online coupling of high-performance liquid chromatography (HPLC). Anal. Chem 83, 9413–7, 10.1021/ac202013s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson CA, Ho E, Strom MB (2016) Chemopreventive properties of 3,3’-diindolylmethane in breast cancer: evidence from experimental and human studies. Nutr. Rev 74, 432–43, doi: 10.1093/nutrit/nuw010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson CA, Newton TA, Graver EJ, Jackson KA, Reid PM, Hartz VL, Cussler EC, Hakim IA (2007) Cruciferous vegetable intake questionnaire improves cruciferous vegetable intake estimates. J. Am. Diet. Assoc 107, 631–43, doi: 10.1016/j.jada.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Turteltaub KW, Dingley KH, Curtis KD, Malfatti MA, Turesky RJ, Garner RC, Felton JS, Lang NP (1999) Macromolecular adduct formation and metabolism of heterocyclic amines in humans and rodents at low doses. Cancer Lett. 43, 149–55, doi: 10.1016/s0304-3835(99)00116-0. [DOI] [PubMed] [Google Scholar]
- Turteltaub KW, Mauthe RJ, Dingley KH, Vogel JS, Frantz CE, Garner RC, Shen N (1997) MeIQx-DNA adduct formation in rodent and human tissues at low doses. Mutat. Res 376, 243–52, doi: 10.1016/s0027-5107(97)00049-3. [DOI] [PubMed] [Google Scholar]
- Uppstad H, Øvrebø S, Haugen A, Mollerup S (2010) Importance of CYP1A1 and CYP1B1 in bioactivation of benzo[a]pyrene in human lung cell lines. Toxicol. Lett 192, 221–8, doi: 10.1016/j.toxlet.2009.10.025. [DOI] [PubMed] [Google Scholar]
- US EPA, (2010). Development of a Relative Potency Factor (RPF) Approach for Polycyclic Aromatic Hydrocarbon (PAH) Mixtures (External Review Draft, Suspended) [WWW Document], U. S. Environ. Prot. Agency Sci. Inventory. URL https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NCEA&dirEntryId=194584, (accessed June 16, 2022). [Google Scholar]
- US EPA, 2017. Toxicological Review of Benzo[a]pyrene [CASRN 50-32-8], https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0136tr.pdf, (accessed June 16, 2022).
- van Poppel G, Verhoeven DT, Verhagen H, Boldbohm RA (1999) Brassica vegetables and cancer prevention. Epidemiology and mechanisms. Adv. Exp. Med. Biol 472, 159–68, doi: 10.1007/978-1-4757-3230-6_14. [DOI] [PubMed] [Google Scholar]
- Villalta PW, Hochalter JB, Hecht SS (2017) Ultrasensitive high-resolution mass spectrometric analysis of a DNA adduct of the carcinogen benzo[a]pyrene in human lung. Anal. Chem 89, 12735–42. doi: 10.1021/acs.analchem.7b02856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DE (2021) Indoles derived from glucobrassicin: Cancer chemoprevention by indole-3-carbinol and 3,3’-diindolylmethane. Front. Nutr 8, 734334, doi: 10.3389/fnut.2021.734334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AW, Chang RL, Levin W, Yagi H, Thakker DR, Jerina DM, Conney AH (1977) Differences in mutagenicity of the optical enantiomers of the diastereomeric benzo[a]pyrene 7,8-diol-9,10-epoxides. Biochem. Biophys. Res. Commun 77, 1389–96, doi: 10.1016/s0006-291x(77)80133-2. [DOI] [PubMed] [Google Scholar]
- Wu S, Yu W (2012) Liquid-liquid extraction of polycyclic aromatic hydrocarbons in four different edible oils from China. Fd. Chem 134, 597–601, doi: 10.1016/j.foodchem.2012.02.155. [DOI] [Google Scholar]
- Wu T-Y, Huang Y, Zhang C, Su Z-Y, Boyanapalli S, Khor TO, Wang H, Lin H, Gounder M, Kagan L, Androulakis IP, Kong A-NT (2015) Pharmacokinetics and pharmacodynamics of 3,3’-diindolylmethane (DIM) in regulating gene expression of phase II drug metabolizing enzymes. J. Pharmacokinet. Pharmacodyn 42, 401–8, doi: 10.1007/s10928-015-9421-5. [DOI] [PubMed] [Google Scholar]
- Zelinkova Z, Wenzl T (2015) The occurrence of 16 EPA PAHs in food – A review. Polycycl. Aromat. Compd 35, 248–84, doi: 10.1080/10406638.2014.918550 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


