Fig. 5.
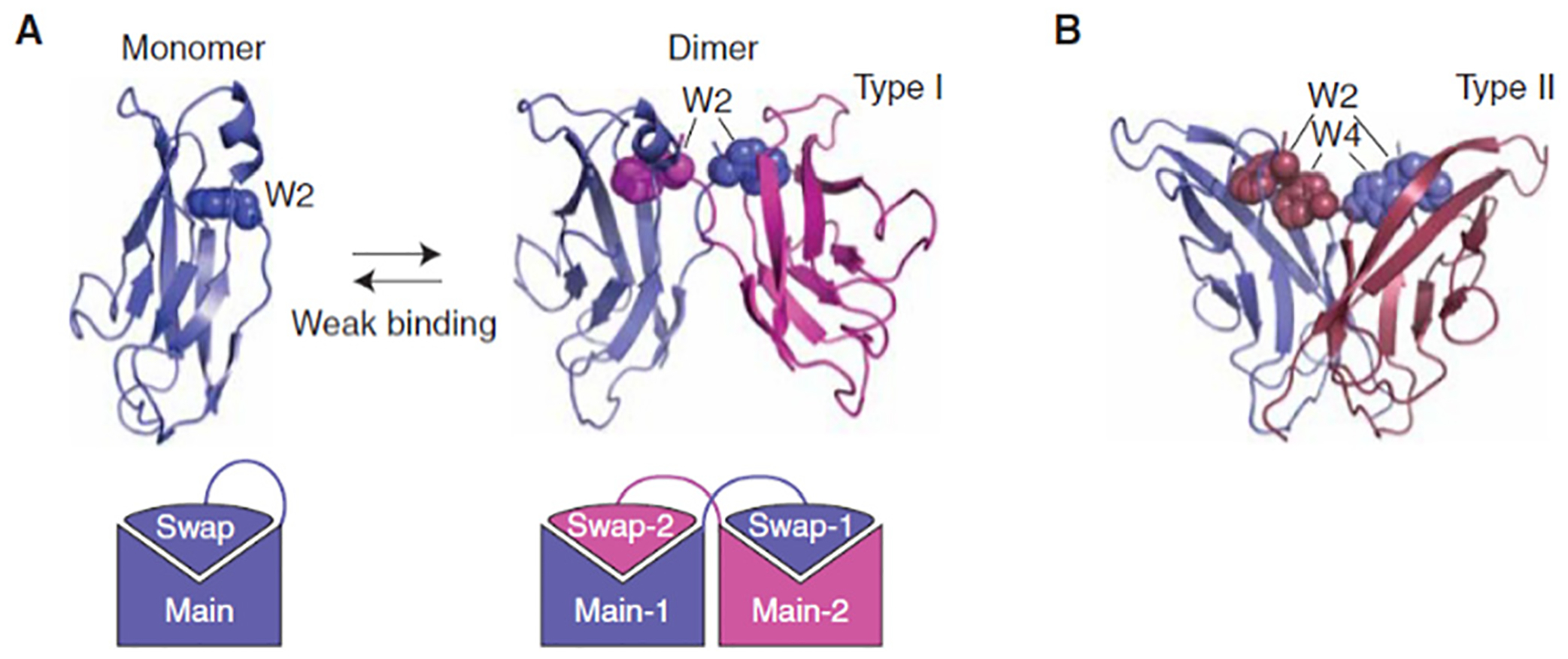
Strand-swap binding by classical cadherins. (A) 3D domain swapping by cadherin EC1 domains. 3D domain swapping provides a simple mechanism for constructing homophilic interfaces. A protein made up of a “main” domain and a “swapping” domain connected by a flexible region can form a “closed” monomer, or a multimer (a dimer in the case of cadherins). These two molecular configurations compete with one another, leading to weak binding affinities. (B) Comparison of strand-swap interfaces of type I and type II classical cadherins. Although the folding topology is identical, these two subfamilies have incompatible binding interfaces. Type II cadherins include two conserved Trp anchor residues, rather than one, and form a hydrophobic interface that runs the length of the EC1 domain. Reproduced with permission.149 Copyright 2009 Cold Spring Harbor Laboratory Press.
