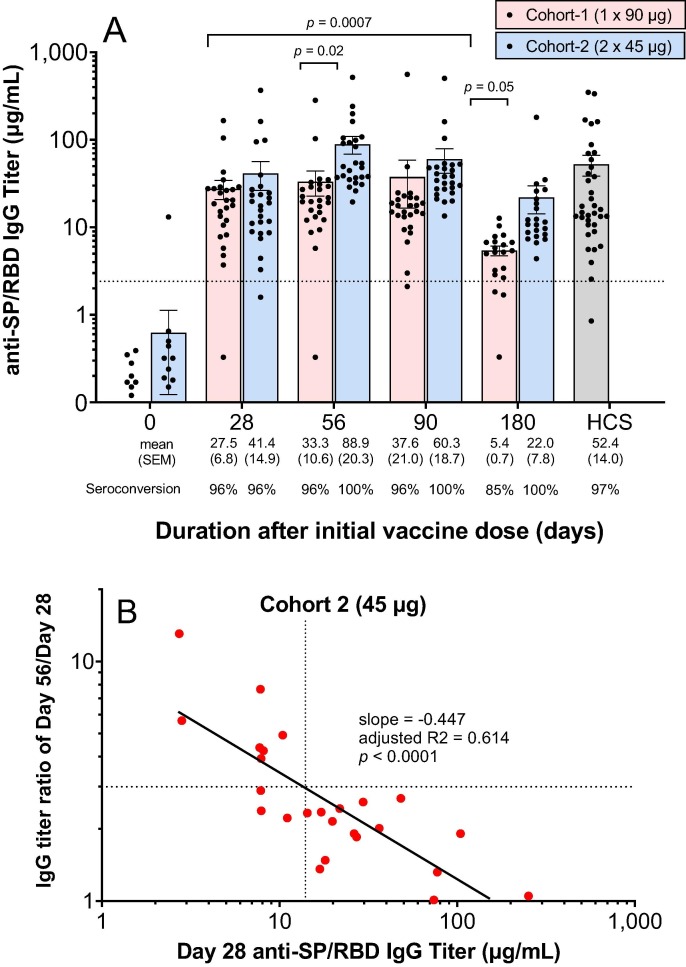
Fig. 2.
AKS-452 immunogenicity: IgG titers. (A) Serum samples were obtained at Days 0, 28, 56, 90 and 180 of the initial vaccine dose and assessed for anti-SP/RBD IgG binding titers via ELISA and presented per subject (all Day 0 samples were < lower limit of quantitation; not shown). Seroconversion was defined as > 2.42 µg/mL IgG (dotted line; derived from validation studies with COVID-19 naïve subject samples; see Methods). HCS titers were used as a comparator for samples from vaccinated subjects. Statistical comparisons between mean values of study day within each cohort and between cohorts were performed using a model with “cohort” and “day” values as fixed effects and a “random subject effect” in which p values were adjusted for multiplicity (Tukey). (B) Correlation between subject’s responsiveness to the second dose (day 56 titer divided by day 28 titer) and the titer on the day of the second dose (i.e., day 28). The dotted lines delineate the approximate day 28 titer that is correlated with a 3-fold response to the second dose at day 56. The solid black line is the linear regression of log-transformed values.