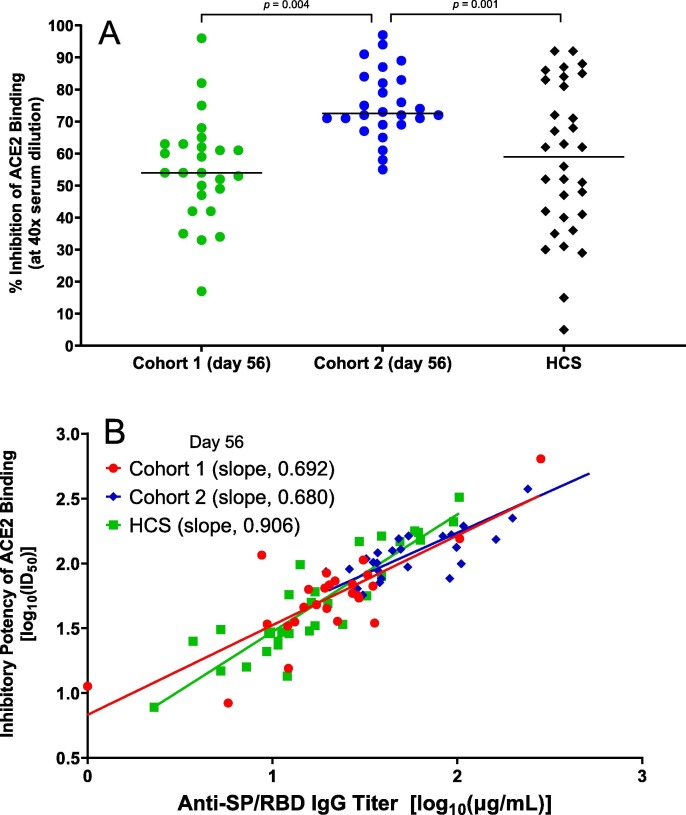
Fig. 3.
AKS-452 immunogenicity:inhibitory potency and IgG Isotype titers. (A) Serum samples were obtained at Day 56 after the initial vaccine dose and assessed at a 1:40 dilution for % Inhibition of recombinant human ACE2 binding to SP/RBD via ELISA and presented per subject (all Day 0 samples were < lower limit of quantitation; not shown). Statistical comparisons between cohorts were performed using a model with “cohort” as a fixed effect in which p-values were not adjusted for multiplicity. (B) Comparison of IgG titer vs. inhibitory potency (inhibitory dilution 50% in ACE2 binding assay, ID50) of Day 56 sera for vaccinated subjects and HCS. Linear regression was performed on log-transformed data; adjusted r2 0.638, p < 0.0001 (cohort 1), adjusted r2 0.680, p ≤ 0.0001 (cohort 2), adjusted r2 0.803, p ≤ 0.0001 (HCS).