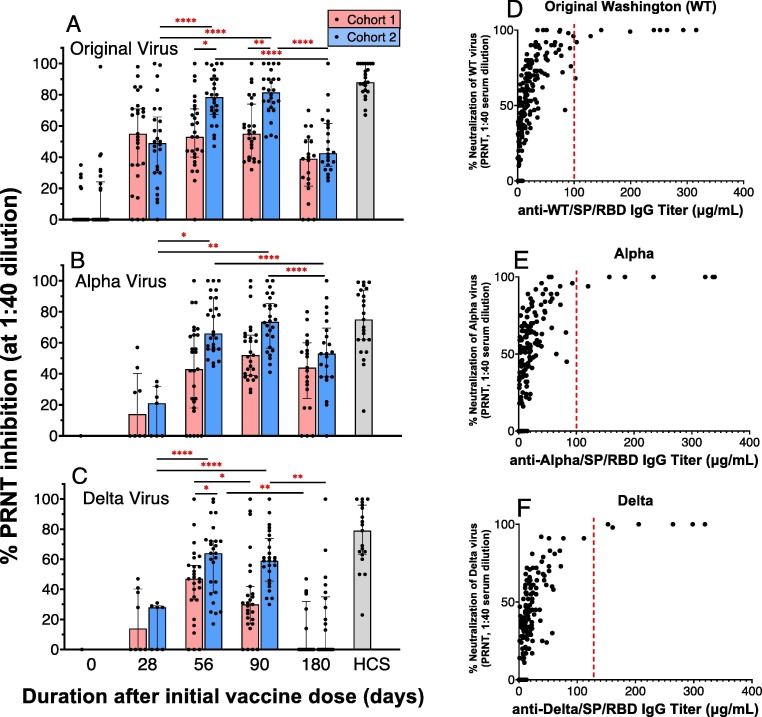
Fig. 6.
AKS-452 serum neutralization of live virus, Plaque Reduction Neutralization Test (PRNT). Serum samples from Cohorts 1 (one 90 µg dose) and 2 (two 45 µg doses) obtained on Days, 0, 28, 56, 90, and 180 of initial vaccine dose were assessed for % neutralization (at 1:40 dilution of serum) of the Original Washington (A), Alpha (B) and Delta (C) live virus strains to infect live VERO E6 cells via the PRNT. For each viral variant (panels A, B, C), statistical comparisons between days “within a cohort” and “between cohorts” were performed using model entailing “cohort” and “day” values as fixed effects and a “random subject effect” using untransformed values in which p values were adjusted for multiplicity (Tukey-Kramer). *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001. PRNT potency values were compared to IgG titers against the respective viral strain SP/RBD (D, E, F) to determine the IgG titer cutoff (∼100 µg/mL) that defined a consistent 100% viral neutralization (red dotted line). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)