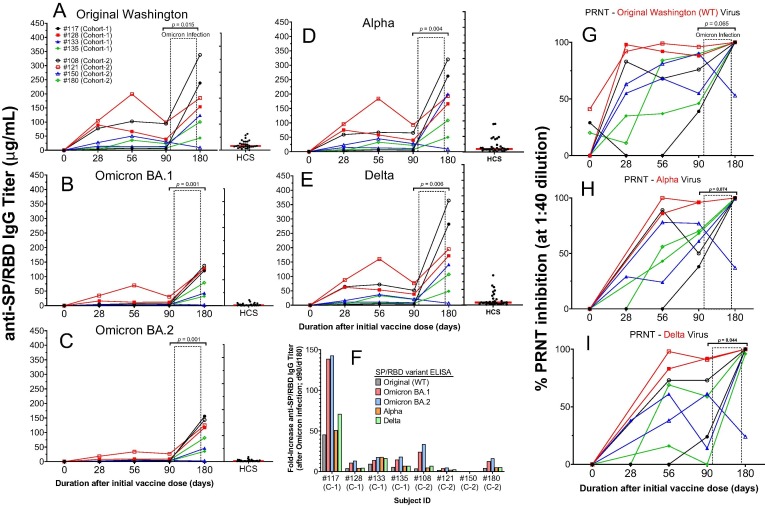
Fig. 8.
Modulation of immunogenicity (IgG titers and neutralization potency) by SARS-CoV-2 Omicron variant infection after day 90 of 8 subjects in the AKS-452 Phase II study. Serum samples were obtained at Day 0, 28, 56, 90, and 180 after the initial vaccine dose from four cohort 1 subjects and four cohort 2 subjects who had acquired SARS-CoV-2 Omicron infection between their day 90 and 180 follow-up visits. Serum samples were assessed for IgG binding titers against SP/RBD from the Original Washington (A), Omicron B.1 (B), Omicron BA.2 (C), Alpha (D), and Delta (E) SARS-CoV-2 strains via ELISA and presented as the mean µg/mL of duplicate tests. The fold-change from day-90 to day-180 titers associated with infection is presented in panel F. Sera were also evaluated for potency to neutralize the Original (G), Alpha (H), and Delta (I) live virus strains from infecting VERO E6 cells via the PRNT. HCS from unvaccinated individuals during the first wave of the SARS-CoV-2 infection (i.e., Original Washington strain) was used as a comparator to samples from vaccinated subjects. For each viral variant and each parameter (IgG titer and PRNT), untransformed values of days 90 and 180 were compared (p values) using an ANOVA model with “day” as a fixed effect and a “random subject effect”.