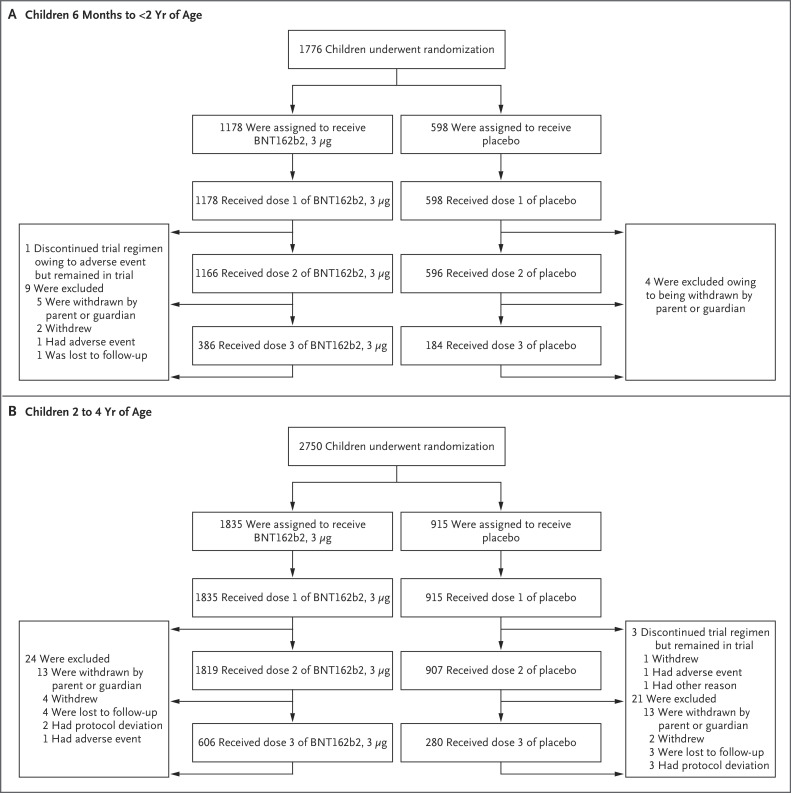
Figure 1. Screening, Randomization, and Vaccine and Placebo Administration in the Phase 2–3 Trial.
At the data-cutoff date (April 29, 2022), the trial was ongoing, and the analyses included all the participants who had received the first dose by March 31, 2022. Therefore, some participants had not received dose 2, and many had not received dose 3 by the data-cutoff date. The phase 2–3 trial was conducted across 65 sites in Brazil, Finland, Poland, Spain, and the United States. Withdrawal by participant describes the inability of the participant to complete trial procedures or visits.