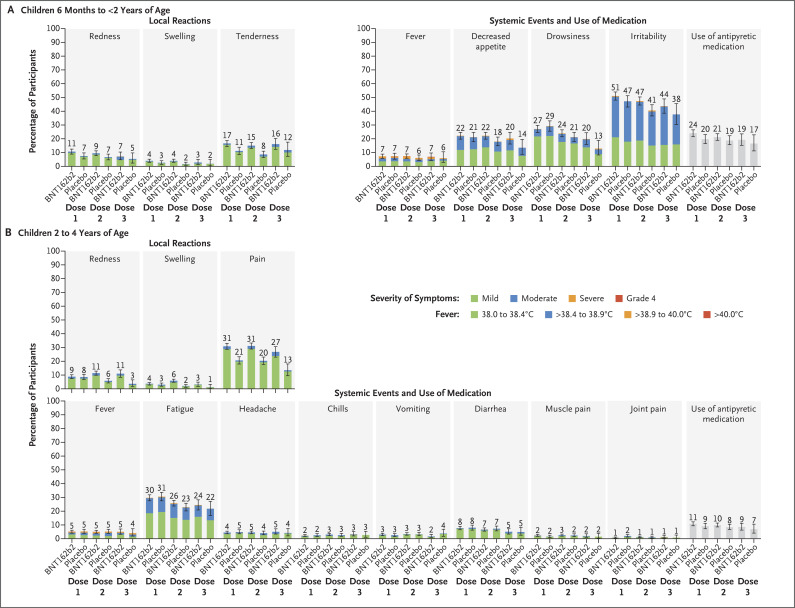
Figure 2. Local Reactions and Systemic Events Reported within 7 Days after Administration of BNT162b2 or Placebo in the Phase 2–3 Trial.
Results are for the blinded, placebo-controlled follow-up period. Shown are local reactions and systemic events after dose 1, dose 2, and dose 3 in BNT162b2 recipients (children 6 months to <2 years of age: dose 1, 1159 to 1173 participants; dose 2, 1137 to 1147 participants; and dose 3, 362 to 365 participants; and children 2 to 4 years of age: dose 1, 1813 to 1825 participants; dose 2, 1772 to 1779 participants; and dose 3, 547 to 552 participants) and in placebo recipients (children 6 months to <2 years of age: dose 1, 591 to 595 participants; dose 2, 590 or 591 participants; and dose 3, 170 participants; and children 2 to 4 years of age: dose 1, 905 to 909 participants; dose 2, 877 or 878 participants; and dose 3, 262 participants). Age-specific severity scales and descriptions for the two age groups are summarized in Table S4. Fever categories are designated in the key. 𝙸 bars represent 95% confidence intervals. The numbers above the bars show the percentage of participants in each group with at least one “yes” or “no” response for the specified event after the specified dose. Four participants 6 months to less than 2 years of age had a fever with a body temperature of more than 40.0°C (3 BNT162b2 recipients: day 1 after dose 1, day 1 after dose 2, and day 3 after dose 3 [1 participant each]; and 1 placebo recipient: day 5 after dose 1); 2 BNT162b2 recipients who had a fever with a body temperature of more than 40.0°C had a concurrent viral infection (roseola on the basis of clinical presentation or concurrent exanthem due to unspecified viral infection). Three BNT162b2 recipients 2 to 4 years of age had a fever with a body temperature of more than 40.0°C (day 2 after dose 1, day 4 after dose 2, and day 6 after dose 2 [1 participant each]), 1 of whom had a clinical presentation suggestive of viral exanthem such as roseola. Missing reactogenicity data were not imputed.