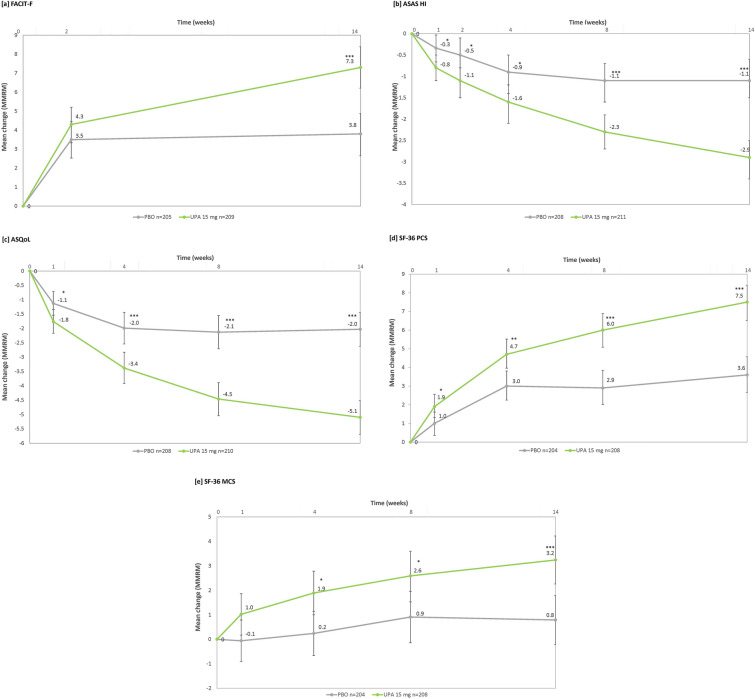
Fig. 2.
Change from baseline through week 14 in a FACIT-F, b ASAS HI, c ASQoL, d SF-36 PCS, e SF-36 MCS (MMRM). MMRM analysis included treatment, visit and treatment-by-visit interaction as fixed factors and baseline value as covariate. Stratification factor hsCRP level (≤ ULN vs. > ULN) were also included in the model. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001. p values nominal except for ASAS HI and ASQoL at week 14, which were ranked endpoints in the multiplicity-controlled analysis. ASAS HI Assessment of SpondyloArthritis international Society Health Index, ASQoL Ankylosing Spondylitis Quality of Life, CI confidence interval, FACIT-F Functional Assessment of Chronic Illness Therapy-Fatigue, hsCRP high-sensitivity C-reactive protein, MCS mental component summary, MMRM mixed effect model for repeated measures, PBO placebo, PCS physical component summary, SF-36 36-Item Short Form Health Survey, ULN upper limit of normal, UPA upadacitinib