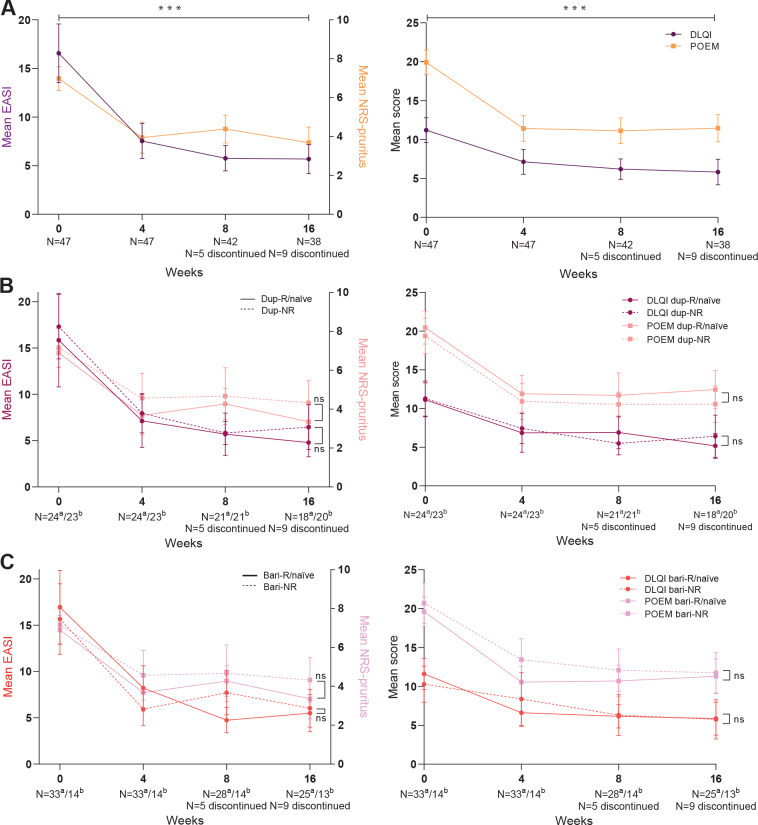
Fig 2.
Primary effectiveness outcomes (mean, 95% confidence interval (95% CI)) of 16 weeks’ treatment with upadacitinib. (A) Outcomes of the total cohort. (B) Outcomes stratified by adup-R/naïve and bdup-NR. (C) Outcomes stratified by abari-R/naïve and bbari-NR. dup-R/naïve: dupilumab responders/naïve patients; dup-NR: dupilumab non-responders; bari-R/naïve: baricitinib responders/naïve patients; bari-NR: baricitinib non-responders; EASI: Eczema Area and Severity Index; NRS: numerical rating scale; DLQI: Dermatology Life Quality Index; POEM: Patient-Oriented Eczema Measure; ns: non-significant. ***p<0.001, bars represent 95% CI.