Highlights
-
•
Cross-sectional survey of adults in buprenorphine treatment for OUD in MA in 2020.
-
•
We investigated health harm perceptions of cigarettes, e-cigarettes and NRT.
-
•
Most participants rated cigarettes as more harmful than e-cigarettes.
-
•
Participants perceived e-cigarettes to be helpful for reducing/quitting cigarettes.
-
•
Participants perceived NRT as safe and helpful for reducing/quitting cigarettes.
Keywords: Electronic cigarette, E-cigarette, Harm perceptions, Opioid use disorder, Buprenorphine, Nicotine replacement therapy
Abstract
Background
Individuals with opioid use disorder (OUD) have a high prevalence of smoking and limited success quitting smoking with existing tools. There is ongoing debate about whether electronic cigarettes (e-cigarettes) may be a viable harm reduction strategy. We sought to determine the potential acceptability of e-cigarettes for cigarette harm reduction among individuals receiving medication treatment for opioid use disorder (MOUD) with buprenorphine. Among individuals receiving MOUD we investigated health harm perceptions of cigarettes, nicotine e-cigarettes, and nicotine replacement therapy (NRT), and perceptions of the helpfulness of e-cigarettes and NRT for quitting cigarettes.
Methods
Cross-sectional telephone survey conducted among adults in buprenorphine treatment at five community health centers in the Boston, MA metropolitan area from February to July 2020.
Results
93% and 63% of participants rated cigarettes and e-cigarettes, respectively, as very or extremely harmful to health, and 62% rated NRT as not to slightly harmful to health. Over half (58%) rated cigarettes as more harmful than e-cigarettes; 65% and 83% perceived e-cigarettes and NRT, respectively, to be helpful for reducing/quitting cigarette use. In bivariate analyses, nicotine e-cigarette users, compared to nonusers, perceived e-cigarettes to be less harmful to health and more often rated e-cigarettes as helpful for reducing/quitting cigarette use (both p<0.05).
Conclusions
This study suggests that Massachusetts patients receiving MOUD with buprenorphine have concerns about the health harms of e-cigarettes yet rate them as helpful tools for reducing or quitting cigarette smoking. Future research is needed to test the efficacy of e-cigarettes for cigarette harm reduction.
1.1. Introduction
Prevalence of smoking among adults with opioid use disorder (OUD) far exceeds the general population (Guydish et al., 2016; U.S. Department of Health and Human Services, 2020; Vlad et al., 2020). Medication treatment for OUD (MOUD) reduces risk of opioid overdose and improves treatment retention (Connery, 2015; Nahvi et al., 2014; Sigmon, 2015; Wakeman et al., 2020; Wen et al., 2018), however, individuals receiving MOUD who continue smoking have substantial risk of tobacco-related mortality (Hser et al., 2017; Hurt et al., 1994, 1996). Even when using smoking cessation treatments, the quit rates for individuals with OUD are substantially lower than the general smoker population (Parker et al., 2020; Vlad et al., 2020).
Electronic cigarettes (e-cigarettes) are devices that heat a nicotine-containing liquid to produce an aerosol that users “vape,” but do not burn tobacco (National Academies of Sciences Engineering and Medicine, 2018). E-cigarette use likely has fewer health risks than smoking and may offer a harm reduction strategy, although the long-term health effects of e-cigarettes are uncertain and debated (Hajek et al., 2019; National Academies of Sciences Engineering and Medicine, 2018; Rigotti, 2020; Pierce et al., 2020; Wang et al., 2021). Regardless, population studies of smokers show substantial interest in and use of e-cigarettes for smoking cessation (Harrell et al., 2015; Hartmann-Boyce et al., 2020; National Academies of Sciences Engineering and Medicine, 2018).
Little is known about how individuals with OUD perceive the health harms or helpfulness of e-cigarettes compared to cigarettes or NRT, information that is essential for assessing the acceptability and uptake of e-cigarettes in this population. It is important to assess health perceptions of e-cigarettes in both methadone and buprenorphine-maintained smokers given that the two medications differ in their pharmacokinetics(Ayanga et al., 2016), which could have differential impact on the nicotine-opioid interaction. Further, it is not clear whether harm perceptions of e-cigarettes in individuals with OUD differ from the general population. Individuals with OUD have comorbidities (e.g., socioeconomic disadvantage)(van Draanen et al., 2020) that could result in differing understanding of health messaging and risk perceptions of e-cigarettes than the general population. Harm perceptions of tobacco products are associated with product initiation in the general population and among individuals in substance use disorder treatment (Campbell et al., 2019; Parker et al., 2018; Popova et al., 2018). One study examined methadone and buprenorphine-maintained smokers’ beliefs about e-cigarettes among those in OUD treatment in Massachusetts (Stein et al., 2015). The authors reported that participants had favorable beliefs about the ability of e-cigarettes to help quit cigarettes and perceived e-cigarettes as less harmful than cigarettes. However, data was collected prior to negative publicity about e-cigarettes (e.g., EVALI/subsequent e-cigarette bans), and the prior study did not provide data on absolute risk perceptions of e-cigarettes nor data on comparative risk of e-cigarettes and NRT or comparative helpfulness for quitting smoking.
To fill these gaps, the present study investigates the harm perceptions of cigarettes, e-cigarettes, and NRT, and perceptions of the helpfulness of e-cigarettes and NRT for reducing or quitting cigarette use among individuals receiving MOUD.
1.2. Methods
1.2.1. Design
The Vaping In Buprenorphine-treated patients Evaluation (VIBE) study methods are published (Streck et al., 2021). VIBE was a cross-sectional telephone survey of patients receiving buprenorphine treatment at five Massachusetts General Hospital community health centers (CHC) in the Boston, Massachusetts area.
1.2.2. Participants and enrollment
Participants were ≥18 years of age, English-speaking, and had received a buprenorphine prescription at the CHCs in the past 2 months. We excluded those who could not consent due to psychiatric/cognitive impairment or deemed inappropriate for participation by their CHC provider. Data were collected from February-July 2020.
1.2.3. Measures
1.2.3.1. Demographic, tobacco, and MOUD characteristics
Participants were asked about age, gender, race/ethnicity, education, past 30-day use of cigarettes and nicotine e-cigarettes, and current use of NRT to quit cigarettes at the time of the survey. Total daily dose of buprenorphine and duration of current buprenorphine treatment were extracted from the health record.
1.2.3.2. Absolute and comparative harm perceptions
For cigarettes, e-cigarettes, and NRT, all participants were asked, “how harmful do you think [product] are/would be to your health?” Response options were “not at all harmful,” “slightly harmful,” “somewhat harmful,” “very harmful,” “extremely harmful,” and “don't know.” Participants were also asked, “Which do you think is more harmful: smoking tobacco cigarettes or vaping nicotine e-cigarettes?” Response options for this question included, “tobacco cigarettes,” “nicotine e-cigarettes,” “both equally harmful,” “neither harmful,” and “don't know.”
1.2.3.3. Perceived helpfulness of e-cigarettes and NRT
All participants were asked to rate how helpful they thought vaping nicotine e-cigarettes and using NRT are for quitting or cutting down on smoking; for both items response options were a 5-point Likert scale from “very unhelpful” to “very helpful,” and included a “don't know” option.
1.2.4. Statistical methods
Bivariate analyses examined the association of participant responses to each absolute harm perception question by current product use status (current use vs. no current use), excluding those who responded “don't know” to each item). The same analytic approach was used to examine the perceived helpfulness of e-cigarettes and NRT for quitting cigarette use. We examined bivariate factors associated with comparative harm of e-cigarettes vs. tobacco cigarettes, excluding participants without an opinion, using chi-squared tests and t-tests. We included variables which differed across comparative harm ratings in bivariate analyses (p<0.15) in a multivariable logistic regression model and generated incidence rate ratios comparing the perceptions that e-cigarettes have greater or equivalent harm to cigarettes vs. cigarettes have higher risk than e-cigarettes. Race was dichotomized (white vs. all other races) for multivariable analyses. Analyses were conducted in STATA version-16 (StataCorp. 2019, College Station, TX).
1.3. Results
1.3.1. Participant characteristics
The VIBE study had a response rate of 43%. Age, gender, and ethnicity of survey respondents and nonrespondents did not differ (p's>0.05). Additional details on recruitment and completion rates have been published (Streck et al., 2021). Among the 222 survey completers, 72% and 31% reported past 30-day cigarette smoking and past 30-day nicotine vaping, respectively (Table 1).
Table 1.
Factors associated with comparative harm of cigarettes vs. nicotine e-cigarettes.
|
Which Is More Harmful: Cigarettes or Nicotine E-cigarettes?a |
|||||
|---|---|---|---|---|---|
| All(N = 222) | Cigarettes are More Harmful than E-cigarettes(N = 120, 58%)a | Nicotine E-Cigarettes are More Harmful or As Harmful as Cigarettes(N = 87, 42%)a | Bivariate P valueb | IRR (95% CI) | |
| Demographic factors | |||||
| Age, years (M±SD) | 46 ± 11.4 | 44 ± 11.2 | 48 ± 11.3 | 0.017 | 1.00 (0.99–1.01) |
| Female (vs. Male) | 108 (49%) | 52 (43%) | 52 (60%) | 0.020 | 1.14 (0.91–1.44) |
| Hispanic, Latino or Spanish origin | 24 (11%) | 10 (8%) | 12 (14%) | 0.21 | |
| Race | 0.048 | ||||
| White | 186 (84%) | 103 (86%) | 72 (83%) | 1.00 (0.73–1.38) | |
| Black | 11 (5%) | 5 (4%) | 4 (5%) | Ref | |
| Other single race | 12 (5%) | 2 (2%) | 8 (9%) | ||
| Multi-race | 13 (6%) | 10 (8%) | 3 (3%) | ||
| Educationc | 0.84 | ||||
| > High school | 110 (50%) | 61 (51%) | 43 (49%) | ||
| ≤ High school or equivalent | 111 (50%) | 59 (49%) | 44 (51%) | ||
| Substance Use & Treatment | |||||
| Cigarette Smoking (past 30d) | 0.049 | ||||
| Yes | 160 (72%) | 91 (76%) | 55 (63%) | 0.94 (0.70–1.27) | |
| No | 62 (28%) | 29 (24%) | 32 (37%) | Ref | |
| E-cigarette Vaping (past 30d) | <0.001 | ||||
| Yes | 69 (31%) | 54 (45%) | 12 (14%) | 0.80 (0.50–1.28) | |
| No | 153 (69%) | 66 (55%) | 75 (86%) | Ref | |
| Dual use (cigarettes and e-cigarettes) (past 30d) | <0.001 | ||||
| Yes | 49 (22%) | 40 (33%) | 6 (7%) | 0.95 (0.54–1.66) | |
| No | 173 (78) | 80 (67%) | 81 (93%) | Ref | |
| Nicotine Use (past 30d) | <0.001 | ||||
| Cigarette only | 111 (50%) | 51 (43%) | 49 (56%) | ||
| Nicotine e-cigarette only | 20 (9%) | 14 (12%) | 6 (7%) | ||
| Dual use | 49 (22%) | 40 (33%) | 6 (7%) | ||
| No use | 42 (19%) | 15 (13%) | 26 (30%) | ||
| Buprenorphine (Bup) Treatment | |||||
| Bup dose (mg), M±SD | 17 ± 7.0 | 19 ± 6.4 | 16 ± 7.3 | 0.002 | 0.99 (0.98–1.01) |
| Years in current bup treatment, M±SD | 2.9 ± 1.34 | 3.1 ± 1.3 | 2.8 ± 1.4 | 0.12 | 0.97 (0.89–1.06) |
Note. Tabled values represent N (column percent) unless otherwise noted and percent's may not add to 100% due to rounding. E-cigarette, electronic cigarette. BUP, buprenorphine. Mg, milligrams. D, day. M, mean. SD, standard deviation. CI, confidence interval. IRR, Incidence rate ratio. Ref, reference group. Race was dichotomized in multivariate analyses (i.e., white vs. all other races).
Response options included tobacco cigarettes are more harmful, nicotine e-cigarettes are more harmful, both are equally harmful, neither are harmful, and don't know. We combined nicotine e-cigarettes are more harmful and both are equally harmful response options into a single category. We excluded the 14 participants who responded “don't know” and the 1 participant who responded “neither are harmful,” and thus present data on 207 of 222 total participants.
p values compare cigarette column to nicotine e-cigarette column using chi-squared tests for categorical variables and t-tests for continuous variables.
1 participant responded “don't know” and was excluded from analyses.
1.3.2. Harm perceptions
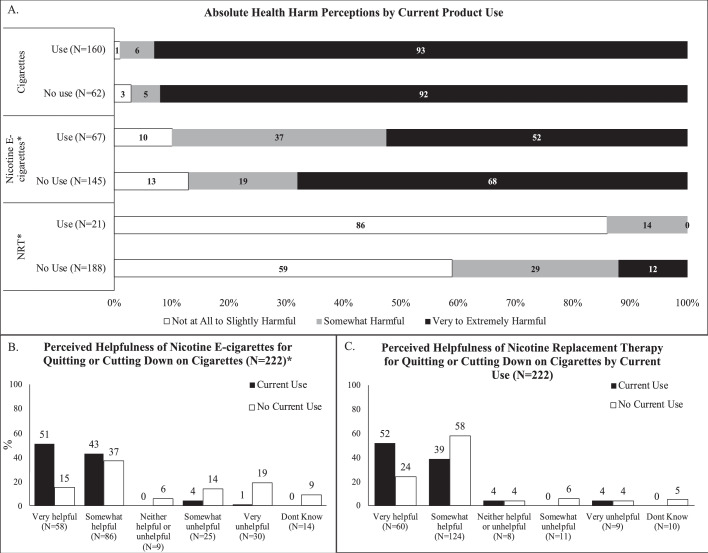
Regarding absolute health harm, 93% of participants rated cigarettes as very or extremely harmful to health. Fewer participants rated e-cigarettes as very or extremely harmful, but the majority (63%) held this perception, while 25% rated e-cigarettes as somewhat harmful and 12% as slightly or not harmful. In contrast, most (62%) participants rated NRT as not at all or slightly harmful to health, while 27% rated NRT as somewhat harmful and 11% as very or extremely harmful. Current e-cigarette users were less likely to rate the products as very or extremely harmful than respondents who were not using e-cigarettes (52% vs. 68%, p = 0.02; Fig. 1, Panel A). Current NRT users were more likely than non-current users to rate NRT as not or slightly harmful (86% vs. 59%, p = 0.02).
Fig. 1.
Absolute health harm perceptions of cigarettes, nicotine e-cigarettes, and NRT by current use of each product (Panel A), perceived helpfulness of e-cigarettes for quitting or cutting down on cigarette smoking by current use of e-cigarettes (Panel B), and perceived helpfulness of NRT for quitting or cutting down on cigarette smoking by current use of NRT (Panel C).
Note: Bar labels represent percentages. For Panel A, values within a bar add to 100% and we only display data for participants who report an opinion. For Panel A, we excluded participants who responded, “don't know” to each item (i.e., 0, 10, and 13 participants responded “don't know” to harm to health of cigarettes, nicotine e-cigarettes, and NRT, respectively). Current use is defined as past 30-day use for cigarettes and e-cigarettes, and current use at the time of the survey for NRT (i.e., Are you currently using the nicotine patch, gum, lozenge, inhaler, or nasal spray to help you quit smoking cigarettes?). For Panels B and C, black bars sum to 100% and white bars sum to 100%.
Key: * denotes p<0.05 for overall chi-squared test of the belief/perception by current product use (yes/no); E-cigarette, electronic cigarette; NRT, nicotine replacement therapy.
Regarding the comparative risk of cigarettes and nicotine e-cigarettes, over half of participants (58%) reported that cigarettes were more harmful than e-cigarettes, and 42% rated e-cigarettes as more harmful or as harmful as cigarettes. In bivariate analyses those who rated nicotine e-cigarettes as having more or equivalent harm to cigarettes were more likely to be older, female, currently smoking cigarettes, not currently vaping nicotine, not currently dual using cigarettes and e-cigarettes, and on a lower dose of buprenorphine. No characteristics remained significantly associated with comparative harm perception ratings in an adjusted logistic regression analysis (Table 1).
1.3.3. Perceived helpfulness of E-cigarettes and NRT
Sixty-five percent of participants rated nicotine e-cigarettes as somewhat or very helpful for quitting or cutting down on cigarettes. Responses differed by past 30-day e-cigarette use (p<0.0001), with current users being more likely to rate e-cigarettes as somewhat or very helpful for reducing or quitting cigarette use compared to non-current users (94% vs. 52%, p<0.0001; Fig. 1, Panel B). In comparison, 83% of participants believed that NRT was somewhat or very helpful for quitting or cutting down on cigarettes, with no differences by current NRT use (91% vs. 82% for current vs. non-current NRT use, p = 0.26; Fig. 1, Panel C).
1.4. Discussion
There is ongoing debate about whether e-cigarettes may be a useful harm reduction tool for smokers including those receiving MOUD, who despite bearing a disproportionate burden of tobacco-related mortality have difficulty quitting smoking with existing tools. Whether e-cigarettes are acceptable to smokers receiving MOUD depends in part on the products’ perceived health harms and perceived helpfulness for smoking reduction or cessation. This study assessed both factors among individuals receiving MOUD. Most participants perceived cigarettes as more harmful than e-cigarettes and rated e-cigarettes as helpful for quitting smoking, suggesting that these products could be acceptable to adults who smoke receiving MOUD. However, caution is warranted because most participants (63%) rated e-cigarettes’ absolute harm to health as very or extremely harmful. E-cigarettes and NRT were rated as similarly helpful for reducing or quitting cigarette use, although e-cigarettes’ absolute health harm was rated as higher than NRT's.
In the single prior study examining risk perceptions of e-cigarettes in adult smokers with OUD in Massachusetts (Stein et al., 2015), 66% of respondents believed e-cigarettes were less harmful than cigarettes, and 74% agreed that e-cigarettes can help individuals quit smoking (Stein et al., 2015). However, that study collected data in 2014, before the 2019 outbreak of acute lung injury associated with vaping of THC (EVALI), which led to increased concern about the health risk of commercial e-cigarettes (Dave et al., 2020). The current study adds to the literature by providing data collected after the EVALI outbreak. The 58% of our sample who rated cigarettes as more harmful than e-cigarettes was lower than the 66% of respondents in the 2014 study (Stein et al., 2015) who held this belief. Our findings are also consistent with a prior study of cigarette smokers admitted to inpatient detoxification for alcohol and/or opioids in New York City in 2015 which reported participant agreement with statements that e-cigarettes help people quit smoking (mean 2.8/5.0) and e-cigarettes are safer than smoking cigarettes (mean 3.1/5.0) with responses on a 1 to 5 scale ranging from “disagree” to “agree” (El-Shahawy et al., 2021).
Nonetheless, a sizable proportion of participants in our sample rated e-cigarettes as harmful and rated e-cigarettes as more or equivalently harmful as cigarettes. Our findings align with recent work in the general population demonstrating increases in perceived harm of e-cigarettes following EVALI (Dave et al., 2020). Additionally, our data were collected shortly after Massachusetts’ subsequent total ban on e-cigarette sales in 2019 (Mass.gov, 2019), which may have elevated perceived harms of e-cigarettes. Regardless, 65% of our sample, including some who reported elevated absolute health risk perceptions of e-cigarettes, also reported that e-cigarettes would be useful for reducing or quitting cigarettes. These findings suggest potential participant agreement with a harm reduction approach where e-cigarettes are perceived as safer than cigarettes and useful for reducing cigarette-related harms, but not free of harms compared to complete abstinence. It is promising that most participants correctly viewed NRT as not harmful and we did not see evidence of misconceptions related to safety of NRT. Unfortunately, data suggest that existing pharmacotherapies produce modest cessation rates in this population and the perceptions of safety and helpfulness of NRT for cessation don't appear to translate into smoking cessation (Miller and Sigmon, 2015; Vlad et al., 2020).
The VIBE study was cross-sectional, precluding making causality inferences. The study was conducted at a single institution in a state with strong e-cigarette restrictions, and results may not generalize beyond our population and state. Finally, it is possible that our sampling procedure may have been unintentionally bias. For example, those who responded to our survey may have been more stable in OUD treatment than those who did not answer (thus more likely to respond).
1.4.1. Conclusions
Most adults in MOUD with buprenorphine perceived cigarettes to be more harmful than e-cigarettes and e-cigarettes and NRT as helpful for reducing or quitting smoking. However, most participants rated e-cigarettes as harmful to health. Further research is needed to test the effectiveness of e-cigarettes for smoking cessation in this population and to subsequently develop accurate health messaging.
Role of funding source
This work was supported by the National Institute on Drug Abuse (NIDA K12 DA043490; Rigotti) and sundry funds provided by Dr. Rigotti.
The funding organization had no role in the study design, collection, analysis, and interpretation of the data, preparation of the manuscript, or decision to submit the manuscript for publication.
Contributors
Design and conduct of the study: Drs. Rigotti and Streck; Data Collection: Ms. Kalagher, Dr. Gupta; Data Analysis: Drs. Streck and Regan; Manuscript Drafting: Drs. Streck and Rigotti; Review of Manuscript for content: Drs. Kalkhoran, Bearnot, Gupta, Regan, Wakeman, and Ms. Kalagher.; All authors have read and approved of the final manuscript.
Declaration of competing interest
Dr. Rigotti receives royalties from UpToDate, has consulted for Achieve Life Sciences, and consulted (without pay) for Pfizer. Dr. Kalkhoran received royalties from UpToDate. Dr. Wakeman receives royalties from UpToDate and has received salary support from OptumLabs, Celero Systems, and Alosa Health. No other authors have conflicts of interest to disclose.
Acknowledgments
None.
References
- Ayanga D., Shorter D., Kosten T.R. Update on pharmacotherapy for treatment of opioid use disorder. Expert Opin. Pharmacother. 2016;17(17):2307–2318. doi: 10.1080/14656566.2016.1244529. [DOI] [PubMed] [Google Scholar]
- Campbell B.K., Le T., Gubner N.R., Guydish J. Health risk perceptions and reasons for use of tobacco products among clients in addictions treatment. Addict. Behav. 2019;91:149–155. doi: 10.1016/j.addbeh.2018.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connery H.S. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv. Rev. Psychiatry. 2015;23(2):63–75. doi: 10.1097/HRP.0000000000000075. [DOI] [PubMed] [Google Scholar]
- Dave D., Dench D., Kenkel D., Mathios A., Wang H. News that takes your breath away: risk perceptions during an outbreak of vaping-related lung injuries. J. Risk Uncertain. 2020;60(3):281–307. doi: 10.1007/s11166-020-09329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shahawy O., Schatz D., Sherman S., Shelley D., Lee J.D., Tofighi B. E-cigarette use and beliefs among adult smokers with substance use disorders. Addic. Behav. Rep. 2021;13 doi: 10.1016/j.abrep.2020.100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Governor baker announces plan to keep vaping product ban in place until December 11th, signs legislation placing new restrictions on vaping. Tobacco Products | Mass.gov. March 15, 2021 https://www.mass.gov/news/governor-baker-announces-plan-to-keep-vaping-product-ban-in-place-until-december-11th-signs (n.d.)Retrievedfrom. [Google Scholar]
- Guydish J., Passalacqua E., Pagano A., Martínez C., Le T., Chun J., Tajima B., Docto L., Garina D., Delucchi K. An international systematic review of smoking prevalence in addiction treatment: smoking prevalence in addiction treatment. Addiction. 2016;111(2):220–230. doi: 10.1111/add.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P., Phillips-Waller A., Przulj D., Pesola F., Myers Smith K., Bisal N., Li J., Parrott S., Sasieni P., Dawkins L., Ross L., Goniewicz M., Wu Q., McRobbie H.J. A randomized trial of E-cigarettes versus nicotine-replacement therapy. New Engl. J. Med. 2019;380(7):629–637. doi: 10.1056/NEJMoa1808779. [DOI] [PubMed] [Google Scholar]
- Harrell P.T., Marquinez N.S., Correa J.B., Meltzer L.R., Unrod M., Sutton S.K., Simmons V.N., Brandon T.H. Expectancies for Cigarettes, E-Cigarettes, and nicotine replacement therapies among E-cigarette users (aka Vapers) Nicotin Tob. Res. 2015;17(2):193–200. doi: 10.1093/ntr/ntu149. Https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann-Boyce J., McRobbie H., Lindson N., Bullen C., Begh R., Theodoulou A., Notley C., Rigotti N.A., Turner T., Butler A.R., Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database Systematic Rev. 2020;10 doi: 10.1002/14651858.CD010216.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser Y.-.I., Mooney L.J., Saxon A.J., Miotto K., Bell D.S., Zhu Y., Liang D., Huang D. High mortality among patients with opioid use disorder in a large healthcare system. J. Addict. Med. 2017;11(4):315–319. doi: 10.1097/ADM.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt R.D., Eberman K.M., Croghan I.T., Offord K.P., Davis L.J., Morse R.M., Palmen M.A., Bruce B.K. Nicotine dependence treatment during inpatient treatment for other addictions: a prospective intervention trial. Alcohol. Clin. Exp. Res. 1994;18(4):867–872. doi: 10.1111/j.1530-0277.1994.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Hurt R.D., Offord K.P., Croghan I.T., Gomez-Dahl L., Kottke T.E., Morse R.M., Melton L.J. Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA. 1996;275(14):1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Miller M.E., Sigmon S.C. Are pharmacotherapies ineffective in opioid-dependent smokers? Reflections on the scientific literature and future directions. Nicotine Tob. Res. 2015;17(8):955–959. doi: 10.1093/ntr/ntv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahvi S., Blackstock O., Sohler N.L., Thompson D., Cunningham C.O. Smoking cessation treatment among office-based buprenorphine treatment patients. J. Subst. Abuse Treat. 2014;47(2):175–179. doi: 10.1016/j.jsat.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences Engineering and Medicine . National Academies Press; 2018. Public Health Consequences of E-Cigarettes. [PubMed] [Google Scholar]
- Parker M.A., Villanti A.C., Quisenberry A.J., Stanton C.A., Doogan N.J., Redner R., Gaalema D.E., Kurti A.N., Nighbor T., Roberts M.E., Cepeda-Benito A., Higgins S.T. Tobacco product harm perceptions and new use. Pediatrics. 2018;142(6) doi: 10.1542/peds.2018-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M.A., Weinberger A.H., Villanti A.C. Quit ratios for cigarette smoking among individuals with opioid misuse and opioid use disorder in the United States. Drug Alcohol. Depend. 2020;214 doi: 10.1016/j.drugalcdep.2020.108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J.P., Benmarhnia T., Chen R., White M., Abrams D.B., Ambrose B.K., Blanco C., Borek N., Choi K., Coleman B., Compton W.M., Cummings K.M., Delnevo C.D., Elton-Marshall T., Goniewicz M.L., Gravely S., Fong G.T., Hatsukami D., Henrie J., Messer K. Role of e-cigarettes and pharmacotherapy during attempts to quit cigarette smoking: the PATH Study 2013-16. PLoS ONE. 2020;15(9) doi: 10.1371/journal.pone.0237938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova L., Owusu D., Weaver S.R., Kemp C.B., Mertz C.K., Pechacek T.F., Slovic P. Affect, risk perception, and the use of cigarettes and e-cigarettes: a population study of U.S. adults. BMC Public Health. 2018;18(1):395. doi: 10.1186/s12889-018-5306-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti N.A. Randomized trials of e-cigarettes for smoking cessation. JAMA. 2020;324(18):1835–1837. doi: 10.1001/jama.2020.18967. [DOI] [PubMed] [Google Scholar]
- Sigmon S.C. The untapped potential of office-based buprenorphine treatment. JAMA Psychiatry. 2015;72(4):395–396. doi: 10.1001/jamapsychiatry.2014.2421. [DOI] [PubMed] [Google Scholar]
- Stein M.D., Caviness C.M., Grimone K., Audet D., Borges A., Anderson B.J. E-cigarette knowledge, attitudes, and use in opioid dependent smokers. J. Subst. Abuse Treat. 2015;52:73–77. doi: 10.1016/j.jsat.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streck J.M., Kalkhoran S., Bearnot B., Gupta P.S., Kalagher K.M., Regan S., Wakeman S., Rigotti N.A. Perceived risk, attitudes, and behavior of cigarette smokers and nicotine vapers receiving buprenorphine treatment for opioid use disorder during the COVID-19 pandemic. Drug Alcohol Depend. 2021;218 doi: 10.1016/j.drugalcdep.2020.108438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . U.S Department of Health and Human Services, Centers for Disease Control and Prevention, National center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2020. Smoking cessation. A report of the surgeon general. [Google Scholar]
- van Draanen J., Tsang C., Mitra S., Karamouzian M., Richardson L. Socioeconomic marginalization and opioid-related overdose: a systematic review. Drug Alcohol. Depend. 2020;214 doi: 10.1016/j.drugalcdep.2020.108127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlad C., Arnsten J.H., Nahvi S. Achieving smoking cessation among persons with opioid use disorder. CNS Drugs. 2020;34(4):367–387. doi: 10.1007/s40263-020-00701-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman S.E., Larochelle M.R., Ameli O., Chaisson C.E., McPheeters J.T., Crown W.H., Azocar F., Sanghavi D.M. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw. Open. 2020;3(2) doi: 10.1001/jamanetworkopen.2019.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.J., Bhadriraju S., Glantz S.A. E-cigarette use and adult cigarette smoking cessation: a meta analysis. Am J Public Health. 2021;111(2):230–246. doi: 10.2105/AJPH.2020.305999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H., Hockenberry J.M., Pollack H.A. Association of buprenorphine-waivered physician supply with buprenorphine treatment use and prescription opioid use in medicaid enrollees. JAMA Netw. Open. 2018;1(5) doi: 10.1001/jamanetworkopen.2018.2943. –e182943. [DOI] [PMC free article] [PubMed] [Google Scholar]