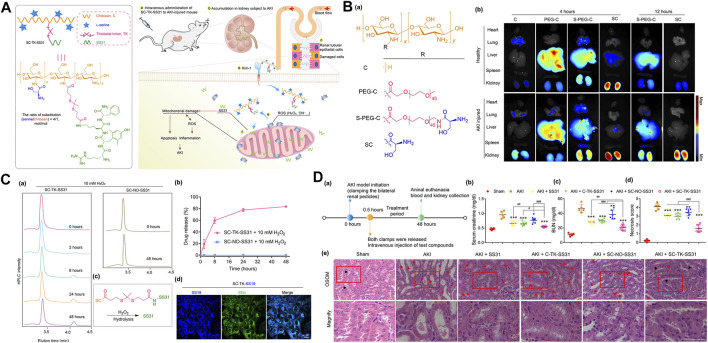
FIGURE 3.
(A)Schematic illustration of the design and structural of ROS cleavable polysaccharide drug precursor SC-TK-SS31, and the principle for targeted AKI treatment; (B) (a) the design and structural of L-serine-modified chitosan (SC) and its analogues, (b) Fluorescent images of heart, lung, liver, spleen and kidney of mice at 4 or 12 h after intravenous injection of various of Cy5-labeled SC and its analogues; (C) (a) The absorption peak (220 nm) of SC-TK-SS31 monitored by HPLC in the presence of 10 mM H2O2, (b) the release curve of SC-TK-SS31 and SC-NO-SS31 dissolved in H2O2 (detected by HPLC), (c) Schematic diagram of SS-31 synthesis (d) Confocal image of HK-2 cells induced by H2O2 incubated with SC-TK-SS19 for 12 h. (D) (a) the schedule for the blood and kidney collection 48 h after intravenous injection of different drugs for AKI mice, (b) Determination of serum creatinine, (c) blood urea nitrogen (BUN) and (d) necrosis score in AKI mice after different treatments, (e) hematoxylin eosin (H&E) staining of renal tissue after different treatment. Adapted with permission from (Sci. Adv. 2020, 6 (41), eabb7422.) (Liu D. et al., 2020).