FIGURE 4.
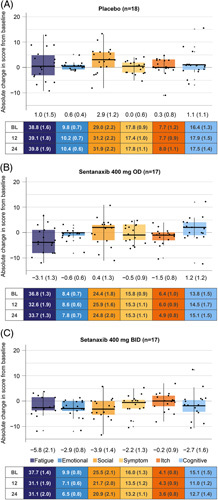
Absolute change in score from baseline to week 24 across the PBC-40 domains in patients with moderate-to-severe fatigue at baseline, by treatment arm. (A) Placebo, (B) setanaxib 400 mg OD, (C) setanaxib 400 mg BID. All patients with moderate-to-severe fatigue at baseline and PBC-40 data available at baseline and Week 24 were included in these analyses (n=52); some patients may have stopped setanaxib treatment before the end of the treatment period. Reduced scores indicate improvements in symptom impact. Box plots show median (central line) and interquartile range; minimum and maximum values are depicted by vertical whiskers, with outliers indicated by crosses (+). Data labels depict mean (SEM) absolute change from baseline in score for each PBC-40 domain. Tables present mean (SEM) absolute scores in each PBC-40 domain at baseline (BL), Week 12 and week 24 by treatment group. Abbreviations: BID, twice daily; OD, once daily; PBC-40, primary biliary cholangitis-40 item questionnaire.
