Abstract
Small nucleolar RNAs (snoRNAs) have been shown to play critical regulatory roles in cancer development. SNORD88C, which located at the intronic region of C19orf48 in chromosome 19q.33 with a 97-nt length was screened through database and snoRNA-sequencing. We firstly verified this snoRNA was up-regulated in tissue and plasma and served as a non-invasive diagnostic biomarker; then confirmed that SNORD88C promoted proliferation and metastasis of NSCLC in vitro and in vivo. Mechanistically, SNORD88C promoted 2′-O-methylation modification at the C3680 site on 28S rRNA and in turn enhanced downstream SCD1 translation, a central lipogenic enzyme for the synthesis of MUFA that can inhibit autophagy by regulating lipid peroxidation and mTOR, providing the novel insight into the regulation of SNORD88C in NSCLC.
Subject terms: Cancer metabolism, Metastasis
Introduction
Non-small cell lung cancer (NSCLC), which accounts for approximately 85% of lung cancer cases worldwide, is a highly malignant tumor and a leading cause of global cancer-related deaths [1]. Despite of continuous advances in the improvement of cancer treatment, only 16% of patients suffering from NSCLC survive within 5 years after diagnosis [2], because at that time approximately two-thirds of patients have presented with metastatic tumors [1]. Therefore, there is an urgent need to deepen our understanding of the mechanism of NSCLC progression and identify effective diagnostic biomarkers and new therapeutic targets.
Small nucleolar RNAs (snoRNAs), mid-size-long noncoding RNAs (ncRNAs), mainly locate within introns of protein-coding genes and long non-coding RNAs [3], and are divided into two categories according their structural basis: H/ACA snoRNAs (SNORAs) and Box C/D snoRNAs (SNORDs), SNORAs form complexes with dyskerin and are responsible for pseudouridynlation of rRNAs whereas SNORDs form the small nucleolar RNA-protein complexes (snoRNPs) with Fibrillarin (FBL) and guide 2´-O-methylation (2´-O-me) of rRNAs [4]. rRNA modification by snoRNAs, especially the 2´-O-me, impacts ribosome biogenesis [5], activity and fidelity of translation [6], then contributes to the regulation of tumorigenesis and progression [7]. For example, SNORD12C/78 guided the 2´-O-me of 28SrRNA at sites G3878 and G4593, then enhanced the translation activity of cancer-related proteins and in turn the malignant phenotype of colorectal cancer [8]. On the contrary, SNORA23 impaired the methylation of G4593 on 28SrRNA and inhibited the progression of hepatocellular carcinoma [9].
Accumulating studies have shown that snoRNAs are abnormally expressed in tumors and play an important role in tumor proliferation and metastasis through a variety of pathways. For example, SNORA42A was highly expressed in colorectal cancer, and promoted cell invasion and metastasis in vivo and in vitro [10]; SNORD12B in esophageal squamous cell carcinoma acted as an oncogene to promote tumor migration and invasion through AKT-mTOR-4EBP1 [11]. Notably, snoRNAs are stably expressed and measurable in body fluids including the blood plasma, serum, and urine of cancer patients, empowering them with the potential as the non-invasive biomarkers for diagnostics of malignancies. For example, plasma SNORD33, SNORD66 and SNORD76 acted as biomarkers for diagnosing lung cancer [12], whereas a six-snoRNAs panel possessed the favorable diagnostic efficiency for renal clear cell carcinoma (RCC) [13]. Our previous studies also demonstrated that SNORD63 and SNORD96A were not only stable in plasma and urinary sediment, but also acted as reliable and promising diagnostic markers for RCC [14].
In this study, we used the database and sequencing results to screen a new oncogene. SNORD88C is located at the intronic region of C19orf48 - the host gene of SNORD88C which is a long non-coding RNA (lncRNA) in chromosome 19q.33 with a 97-nt length. We firstly verified this snoRNA was up-regulated in tissue and plasma and served as a non-invasive diagnostic biomarker; then confirmed that SNORD88C promoted proliferation and metastasis of NSCLC in vitro and in vivo. Importantly, our data supported SCD1 was the direct downstream of SNORD88C. SNORD88C enhanced its translation activity though guiding 2´-O-me of 28S rRNA, in turn inhibited autophagy to promote the migration and invasion of NSCLC cells, providing the novel insight into the regulation of SNORD88C in NSCLC.
Results
SNORD88C is up-regulated in NSCLC and serves as a non-invasive diagnostic biomarker
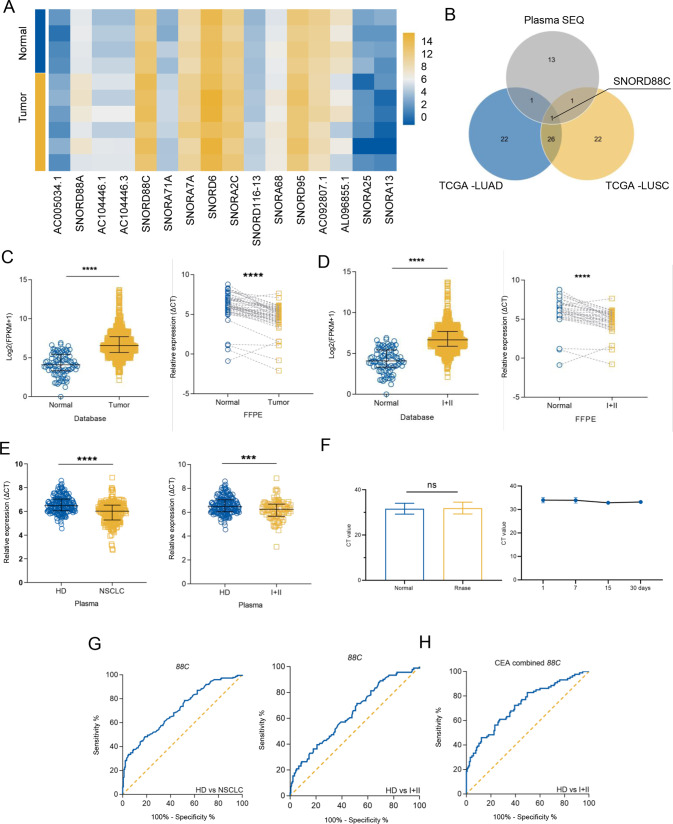
To identify snoRNAs aberrantly expressed in NSCLC, we analyzed the differential expressions of snoRNAs between tumor tissues (including lung adenocarcinoma (LUAD) and lung squamous carcinoma (LUSC)) and para-tumoral tissues in SNORic database from TCGA, the top 50 genes with the most significant difference were represented by heat map (Fig. S1A, B). Next, plasma from 4 healthy donors and 6 NSCLC patients were subjected to snoRNA sequencing, the volcano map demonstrated the differential snoRNAs (Fig. S1C), among which 12 up-regulated and 4 down-regulated snoRNAs were selected due to P < 0.05 (Fig. 1A). Finally, the differential snoRNAs mentioned above were subjected to VENN diagram analysis (Fig. 1B), and only SNORD88C was overlapped and chosen as the candidate.
Fig. 1. SNORD88C is up-regulated in NSCLC and serves as a non-invasive diagnostic biomarker.
A Though the snoRNAs-sequencing in plasma from NSCLC patients and donors, the differential snoRNAs was shown by heatmap. B The top 50 snoRNAs with the largest fold change in the LUAD and LUSC data sets from TCGA and the statistically differential snoRNAs in the sequencing results were analyzed using the VENN diagram, in which only SNORD88C was overlapped. C, D SNORD88C was significantly increased in NSCLC and early NSCLC tissues from TCGA database and FFPE samples, compared with para-tumor tissues (E) SNORD88C was significantly increased in the plasma of NSCLC and early NSCLC patients, compared with the plasma of donors. F SNORD88C was stably expressed in plasma treated with RNase (left) or stored at −80 °C at time-points of 1, 7, 15, 30 days (right). G The diagnostic efficacy of plasma SNORD88C for NSCLC (left) and early-NSCLC (right). H The diagnostic efficacy of SNORD88C combined with CEA for early-NSCLC. Two-tailed paired t-test, two-tailed unpaired t-test, Mann-Whitney test, one-way analysis of variance (ANOVA) or Kruskal-Wallis test; ns, no significance; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
We further verify the upregulation of SNORD88C in NSCLC. As shown in Fig. 1C, SNORD88C was significantly elevated in the tumor tissues compared to the para-tumor tissues in both TCGA data (left, 989 vs 91) and FFPE detection (right, 48 NSCLC and paired para-tumor tissues). We also analyzed the relationship between SNORD88C and clinicopathological parameters, which was related to the histopathologic type, T stage, and N stage (Fig. 2SA–C, Tables S1 and 1). Moreover, as shown in Fig. 1D up-regulated SNORD88C expression was also observed in early-stage NSCLC (I + II) compared with the normal tissue in both TCGA data (left, I + II = 784 vs 91) and FFPE detection (right, I + II = 34 pairs), implying its role in the the occurrence and development of NSCLC.
Further verification of SNORD88C level in the plasma from 178 healthy donors and 224 NSCLC patients (including I + II = 87) revealed its upregulation in NSCLC and early-stage NSCLC, consistently (Fig. 1E), which was significantly correlated with tumor size, and tumor progression, TNM stages (Fig. 2SD–I, Table 2). Notably, SNORD88C predominately expressed in vesicle-depleted plasma other than in exosomes and MVs (Fig. S2J) and was stable in the plasma as evidence from no obvious change observed after RNase A treatment or storage for 30 days (Fig. 1F). Meanwhile, ROC curves analysis was performed to investigate diagnosis efficacy, possessing the area under curve (AUC) for NSCLC and early-stage NSCLC was 0.7097 and 0.6406, respectively (Fig. 1G). Carcinoembryonic antigen (CEA), a traditional biomarker generally accepted clinically, possesses the poor clinic diagnostic efficiency for early-NSCLC. Nevertheless, the AUC of CEA for early-NSCLC was significantly elevated from 0.6650 to 0.7279 when combined with SNORD88C (Fig. 1H and Fig. S2K). Taken together, SNORD88C is up-regulated in NSCLC and serves as a non-invasive diagnostic biomarker.
Table 2.
Characteristics of NSCLC patients for SNORD88C in plasma.
| Parameters | Samples, n | SNORD88C expression | |
|---|---|---|---|
| Δ Ct Median (interquartile range) | P-value | ||
| Age (years) | |||
| <62 | 105 | 6.050 (5.280–6.455) | 0.7496 |
| ≥62 | 119 | 5.990 (5.200–6.570) | |
| Gender | |||
| Male | 134 | 6.080 (5.165–6.630) | 0.5101 |
| Female | 90 | 5.990 (5.368–6.368) | |
| Smoking | |||
| No | 111 | 6.070 (5.440–6.460) | 0.6028 |
| Yes | 111 | 5.910 (5.090–6.670) | |
| Not available | 2 | ||
| Drinking | |||
| No | 168 | 6.110 (5.400–6.485) | 0.3914 |
| Yes | 56 | 5.865 (4.970–6.685) | |
| Histology | |||
| AC | 158 | 6.005 (5.285–6.443) | 0.4673 |
| SCC | 47 | 5.880 (5.200–6.690) | |
| Others | 19 | ||
| Tumor size | |||
| ≤6 cm3 | 80 | 6.105 (5.560–6.565) | 0.0490 |
| >6 cm3 | 80 | 5.905 (5.083–6.438) | |
| Not available | 64 | ||
| T stage | |||
| Tis | 8 | 6.175 (5.568–6.782) | 0.0142 |
| T1 | 68 | 6.124 (5.928–6.321) | |
| T2 | 70 | 5.877 (5.651–6.102) | |
| T3 | 17 | 5.625 (5.055–6.194) | |
| T4 | 42 | 5.569 (5.305–5.832) | |
| TX | 19 | ||
| LN meta. | |||
| N0 | 97 | 6.230 (5.665–6.615) | 0.0008 |
| N1 | 17 | 6.290 (5.570–6.695) | |
| N2 | 53 | 5.800 (5.060–6.360) | |
| N3 | 40 | 5.420 (4.873–6.285) | |
| NX | 17 | ||
| Distant meta. | |||
| M0 | 131 | 6.160 (5.550–6.600) | 0.0023 |
| M1 | 91 | 5.790 (5.050–6.360) | |
| MX | 2 | ||
| TNM stage | |||
| 0 | 7 | 6.160 (5.630–6.840) | 0.0100 |
| I | 69 | 6.250 (5.750–6.685) | |
| II | 15 | 6.110 (5.610–6.690) | |
| III | 39 | 5.880 (5.090–6.420) | |
| IV | 92 | 5.800 (5.055–6.368) | |
| Not available | 2 | ||
Bold values represent statistical significance p < 0.05.
SNORD88C promotes proliferation of NSCLC in vitro and in vivo
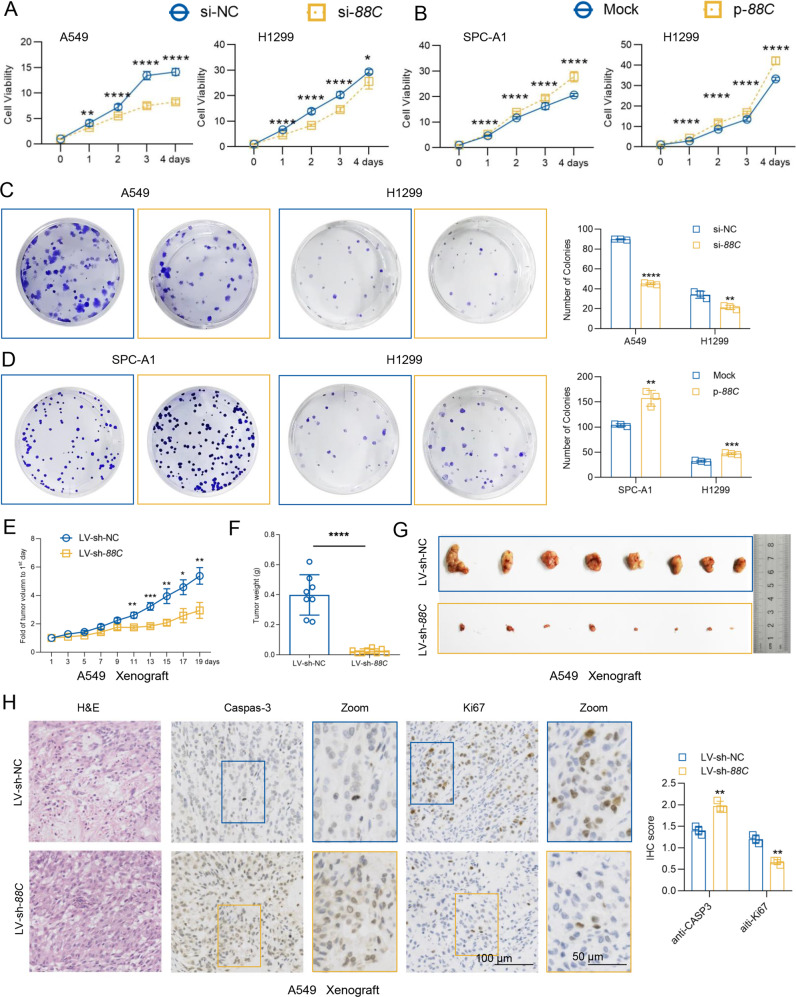
To elucidate the role of SNORD88C in NSCLC progression, we overexpressed or downregulated SNORD88C in three NSCLC cell lines (A549, H1299, and SPC-A1) according to their background expression (Fig. S3A). we also verified the transfection efficiency by q-PCR (Fig. S3B–F), as well as the effect of SNORD88C silencing on its host gene C19orf48 (Fig. S3G–L), neither transient nor steady silencing of SNORD88C affected the expression of C19orf48. Next, CCK-8 and colony formation analysis were employed to determine the effect of SNORD88C on cell proliferation. As shown in Fig. 2A, SNORD88C knockdown significantly suppressed cell viability of A549 and H1299 cells; On the contrary, forced SNORD88C expression enhanced growth in SPC-A1 and H1299 cells (Fig. 2B). Consistently, A549 and H1299 cells with silencing SNORD88C showed suspended clonogenicity, whereas SNORD88C overexpression caused obvious increase in the colony-formation ability of SPC-A1 and H1299 cells (Fig. 2C, D).
Fig. 2. SNORD88C promotes proliferation of NSCLC in vitro and in vivo.
CCK8 (A, B) and clone formation (C, D) assays showed that knockdown of SNORD88C inhibited proliferation (A) and clonogenicity (C) in A549 and H1299 cells, while overexpression of SNORD88C in SPC-A1 and H1299 cells showed the opposite effect (B, D). In the A549 tumor transplantation model, the growth rate (E) of xenograft was slower, and the weight (F) and volume (G) decreased in LV-sh-SNORD88C compared with the control. H Immunohistochemical staining showed that the expression of Caspas-3 increased and the expression of Ki67 decreased in the tumor tissue of LV-sh-SNORD88C group compared with the control. Scale bar indicates 50 μm and 100 μm.; Two-tailed unpaired t-test, Mann-Whitney test, one-way analysis of variance (ANOVA) or Kruskal-Wallis test; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To determine the effects of SNORD88C on NSCLC proliferation in vivo, we established xenograft nude mouse model using A549 cells with SNORD88C stable knockdown (LV-sh-SNORD88C) or not (LV-sh-NC) (Fig. S3F). As expected, xenografts in LV-sh-SNORD88C group grew significantly slower than those in control group (Fig. 2E) and showed a significant decrease in tumor weight and volume compared to the control (Fig. 2F, G). Immunohistochemistry staining for Caspase-3 and Ki67 revealed that LV-sh-SNORD88C promoted apoptosis but inhibited proliferation in A549 xenografts (Fig. 2H). In summary, SNORD88C acts as a regulator of proliferation in NSCLC in vitro and in vivo.
SNORD88C promotes NSCLC migration and invasion in vitro and in vivo
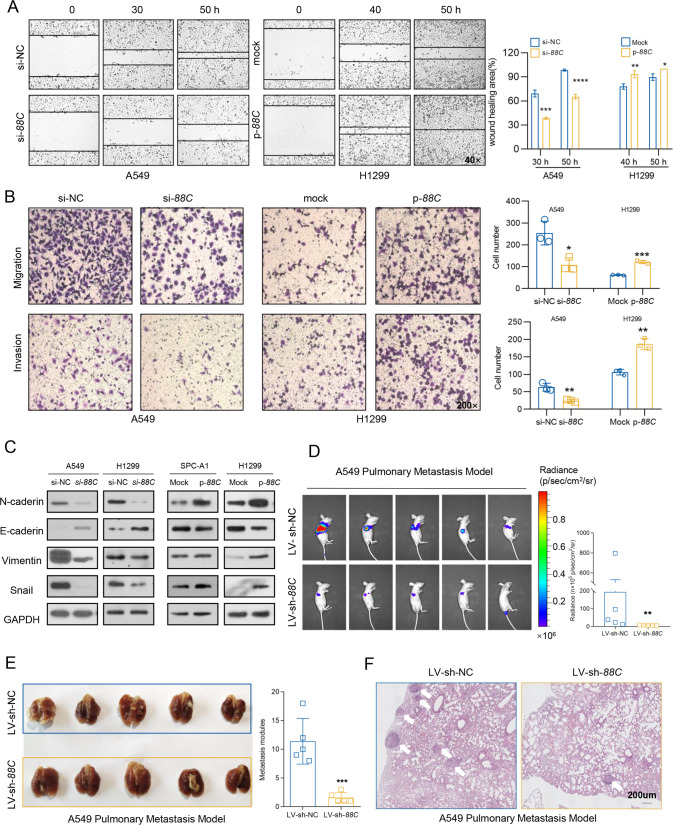
As described above, the elevated SNORD88C was related with tumor progression in the TCGA database and plasma detection, thus, we next determined its role in migration and invasion. The wound healing assays revealed that the mobility was dramatically inhibited by SNORD88C knockdown in A549 and H1299 cells, but promoted by its overexpression in H1299 and SPC-A1 cells (Fig. 3A and S4A). Moreover, A549 and H1299 cells displayed significantly decreased migration and invasion abilities after silencing SNORD88C expression, but these abilities were stimulated remarkably by overexpressed SNORD88C (Figs. 3B and S4B). Considering the crucial role of EMT in invasion and migration, we then detected effect of SNORD88C on EMT-related protein expression. As shown in Fig. 3C, SNORD88C knockdown increased E-cadherin but suppressed N-cadherin, Vimentin, and Snail in A549 and H1299 cells, whereas SNORD88C overexpression displayed the opposite effect in SPC-A1 and H1299 cells.
Fig. 3. SNORD88C promotes NSCLC migration and invasion in vitro and in vivo.
A, B Wound healing, migration and invasion assays showed that knockdown of SNORD88C in A549 cells inhibited the motility, migration and invasion of NSCLC, while overexpression of SNORD88C in H1299 cells showed the opposite effect. Original magnification for wound healing, ×40; Original magnification for migration and invasion, ×200. C Western blot showed that SNORD88C knockdown decreased the expression of N-cadherin, Vimentin, and Snail and increased E-cadherin expression in A549 and H1299, while overexpression of SNORD88C showed the opposite expression for these proteins in SPC-A1 and H1299 cells. D, E In A549 pulmonary metastasis model the luciferase signal intensities in the LV-sh-SNORD88C group was remarkably lower than that in the control. F H&E staining showed that metastatic nodules in the lung tissue invaded the pulmonary capsule in the LV-sh-NC group, but not in the LV-sh-SNORD88C. Scale bar indicates 200 μm; Two-tailed unpaired t-test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Furthermore, we established the pulmonary metastasis model by injecting A549 cells with stably SNORD88C knockdown and firefly luciferase expression to the tail vein of the nude mice. As expected, in vivo bioluminescence imaging (BLI) showed the luciferase signal intensities in the LV-sh-SNORD88C group was remarkably lower than that in the control (Fig. 3D). After lung tissues separation, fewer metastatic loci occurred in the murine lungs in the LV-sh-SNORD88C group than those in the control group (Fig. 3E). Besides, H&E staining demonstrated some metastatic loci invaded the lung capsule in the LV-sh-NC group but none was observed in the LV-sh-SNORD88C group (Fig. 3F). These results demonstrate that SNORD88C promotes NSCLC migration and invasion in vitro and in vivo.
SNORD88C-attenuated autophagy is required for its promotion of migration and invasion
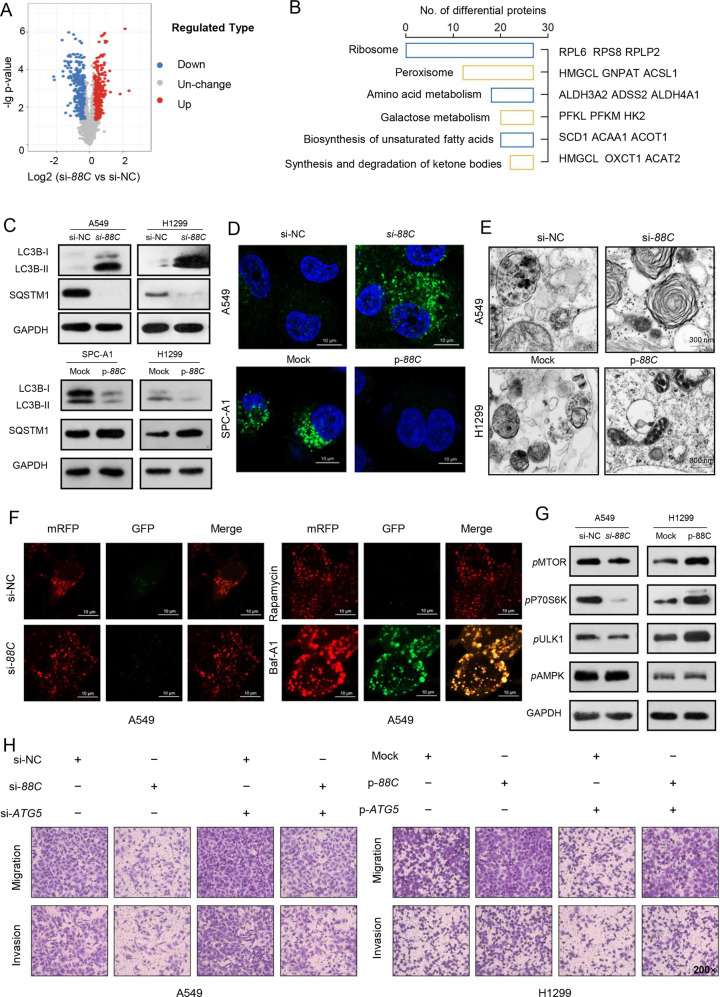
To explore the downstream target genes of SNORD88C, LC-MS/MS-based TMT-labeled quantitative proteomic was employed in the H1299 with silencing SNORD88C or negative control. As shown in Fig. 4A, a total of 384 up-regulated proteins and 369 down-regulated proteins were presented at the volcano map due to p-value ≤ 0.05 and FC ≥ 1.2, most of which were enriched in metabolism related pathways (Fig. 4B).
Fig. 4. SNORD88C-attenuated autophagy is required for its promotion of migration and invasion.
A The volcano map showed that the differential proteins analyzed by LC-MS/MS-based TMT-labeled quantitative proteomic in SNORD88C-knockdown H1299 cells and the control group. (p ≤ 0.05 and FC ≥ 1.2) B The differential proteins enrichment pathways. C–E WB, immunofluorescence labeled by anti-LC3 antibody as well as transmission electron microscopy showed that autophagy was activated in SNORD88C-knockdown NSCLC cells, but inhibited in SNORD88C-overexpression NSCLC cells. Scale bar indicates 10 μm and 300 μm. F A549 cells expressing mRFP-GFP-LC3 were transfected with si-NC or si-88C, or treated with 1 nM rapamycin or 1 nM Baf-A1 for 24 h and imaged by confocal microscopy. Scale bars: 10 μm. G WB assay showed that the phosphorylation levels of MTOR, P70S6K, and ULK1 were decreased in SNORD88C-knockdown A549 cells, while the phosphorylation levels of these proteins were enhanced in SNORD88C-overexpression H1299 cells. The AMPK phosphorylation level was not affected by SNORD88C knockdown or overexpression. H ATG5 silencing could reverse the inhibitory effect of SNORD88C knockdown on the migration and invasion of A549 cells (left). ATG5 overexpression could abolish the promotion of SNORD88C overexpression on the migration and invasion of H1299 cells (right). Original magnification, ×200. Two-tailed unpaired t-test, **P < 0.01.
Autophagy, as it is routinely referred to, allows cells to maintain metabolic sufficiency and survive under conditions of nutrient stress and can be regulated by multiple metabolic pathways. Hence, we further detected the effect of SNORD88C on autophagy. As shown in Fig. 4C, autophagy was activated by SNORD88C knockdown both in A549 and H1299 cells whereas SNORD88C overexpression displayed the inhibitory effect on autophagy in SPC-A1 and H1299 cells. Accordingly, SNORD88C-attenuated autophagy was also verified by immunofluorescence labeled by anti-LC3 antibody to show the formation of autophagosomes, which demonstrated an increase in the number of autophagosomes by SNORD88C knockdown but a decrease by SNORD88C overexpression (Figs. 4D, S5A, B). Next, this phenomenon was further confirmed by transmission electron microscope (TEM). Ultrastructurally, SNORD88C knockdown significantly increased whereas SNORD88C overexpression decreased the autophagy vacuoles in numbers as shown in Figs. 4E, S5C, D. Furthermore, we confirmed the role of SNORD88C in autophagy flux via transfection of adenovirus-mediated mRFP-GFP-LC3 vector, which labelled autophagosomes yellow because of superposition of GFP and mRFP signals, and autolysosomes red as the low lysosomal pH quenches the GFP signal [15]. As shown in Figs. 4F, S5E, most of the puncta lost the GFP signal and retained the mRFP signal in SNORD88C silencing or overexpressed cells like that in the rapamycin-treated cells, but unlike that in cells treated by Baf-A1, quenching of the GFP was significantly diminished, indicating that SNORD88C demonstrated little impact on autophagy flux. Next, we investigated whether the mTOR/ULK1 signaling contributes to SNORD88C-attenuated autophagy. As shown in Fig. 4G, SNORD88C knockdown led to downregulation of phosphorylations of MTOR and its downstream proteins P70S6K and ULK1 in A549, while phosphorylations of these proteins were increased after SNORD88C overexpression in H1299. Notably, AMPK phosphorylation was not affected by SNORD88C, indicating SNORD88C regulated mTOR/ULK1 signaling independent on AMPK (Fig. 4G).
To provide a direct link between SNORD88C-attenuated autophagy and it-induced migration and invasion of lung cancer cells, NSCLC cells were transfected with ATG5 siRNA (si-ATG5) and plasmid (p-ATG5) (Figs. 4H, S5F), as well as treated with 3-Methyladenine (3-MA) or rapamycin (Fig. S5G, H) followed by migration and invasion detection. si-ATG5 and 3-MA could abolish the suppressive effect on migration and invasion caused by SNORD88C silencing in A549 cells, whereas the metastatic effect of H1299 enhanced by SNORD88C overexpression was reversed at least partially by p-ATG5 or rapamycin addition. Overall, these results support that SNORD88C suppresses autophagy and then promote the migration and invasion of NSCLC cells.
SNORD88C inhibits autophagy via upregulating SCD1 protein expression
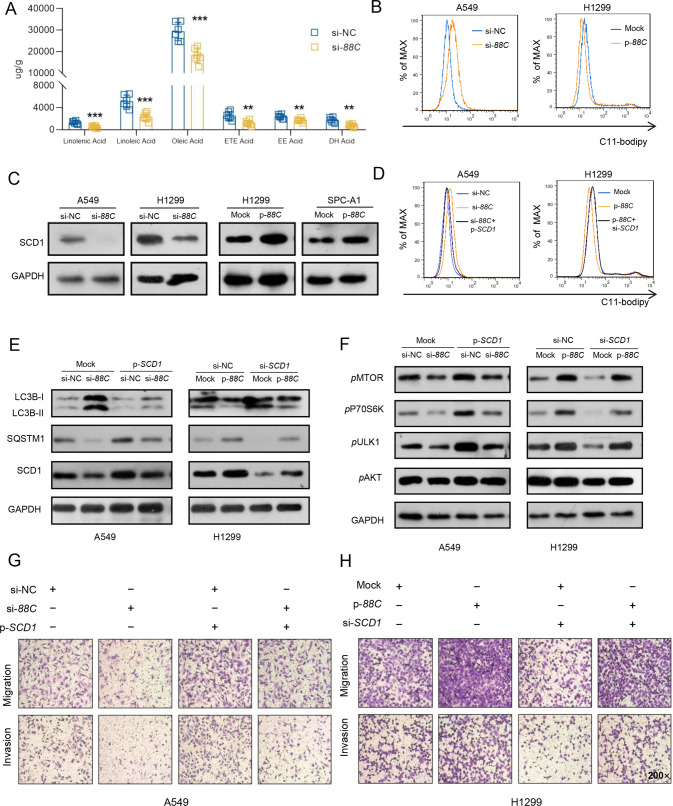
To figure out the metabolites involved in SNORD88C-attenuated autophagy, GC-MS-based metabolomics targeting fatty acids were performed in H1299 cells with SNORD88C knockdown since pathway of unsaturated fatty acids biosynthesis was enriched in above LC-MS/MS-based TMT-labeled quantitative proteomic (Fig. 4B). As shown in Figs. 5A, S6A, many kinds of unsaturated fatty acids were decreased in SNORD88C knockdown cells compared to the control, among which the decease of oleic acid (OA), a monounsaturated fatty acid (MUFA), seemed more significant, indicating SNORD88C played a crucial role in lipid accumulation and peroxidation. Hence, C11-bodipy staining was employed, and flow cytometry analysis showed that lipid peroxidation was promoted by silencing SNORD88C but inhibited by its overexpression (Figs. 5B, S6C), consistent with the result that SNORD88C could inhibit autophagy.
Fig. 5. SNORD88C inhibits autophagy via upregulating SCD1 protein expression.
A GC-MS-based metabolomics targeting fatty acids showed many kinds of unsaturated fatty acids were decreased in SNORD88C-knockdown H1299 cells compared to the control group. B Flow cytometry showed that through C11-bodipy staining, lipid peroxidation was promoted in SNORD88C-knockdown A549 cells, while inhibited in SNORD88C-overexpression H1299 cells. C WB showed that SCD1 expression was decreased in SNORD88C-knockdown A549 and H1299 cells, and increased in SNORD88C-overexpression SPC-A1 and H1299 cells. D The promotion of lipid peroxidation by SNROD88C knockdown in A549 cells and the inhibition of lipid peroxidation by SNROD88C overexpression in H1299 cells could be reversed by SCD1 overexpression and knockdown. E In A549, the overexpression of SCD1 reversed the increase of LC3B and the decrease of SQSTM1 caused by SNORD88C silencing. In H1299, knocking down SCD1 abolished the decrease of LC3B and the increase of SQSTM1 induced by SNORD88C overexpression. F SCD1 overexpression reversed the decreased phosphorylation of MTOR, P70S6K, and ULK1 caused by SNORD88C knockdown in A549. SCD1 knockdown abolished the increased phosphorylation of these proteins induced by SNORD88C overexpression in H1299. G In A549, SCD1 overexpression could reverse the inhibitory effect of SNORD88C knockdown on migration and invasion. H In H1299, SCD1-knockdown could abolish the promoting effect of SNORD88C overexpression on the migration and invasion. Original magnification, ×200. Two-tailed unpaired t-test, **P < 0.01, ***P < 0.001.
Stearoyl-CoA desaturase 1 (SCD1) is a central lipogenic enzyme for the synthesis of MUFA, which also exhibited the most significant change in above LC-MS/MS-based TMT-labeled quantitative proteomic (Fig. S6B) after silencing SNORD88C. Importantly, western blot assays successfully validated SNORD88C positively regulated SCD1 protein level, which was decreased by SNORD88C knockdown in A549 and H1299 cells but increased significantly after SNORD88C overexpression in H1299 and SPC-A1 cells (Figs. 5C, S6D). Accordingly, flow cytometry analysis showed that the promotion of silencing SNORD88C, as well as the inhibitory effect of SNORD88C overexpression on lipid peroxidation could be successfully reversed by SCD1 overexpression and knockdown, respectively (Figs. 5D, S6E), suggesting SNORD88C inhibited lipid peroxidation via upregulating SCD1 protein expression.
Furthermore, we explored the role of SCD1 in SNORD88C-attenuated autophagy. As shown in Figs . 5E, S6F, overexpression of SCD1 could restore autophagy activation by SNORD88C knockdown in A549 cells, as evidence from the decrease in LC3B expression in the SNORD88C-knockdown plus SCD1-overexpression group; Consistently, SCD1 silencing relieved autophagy inhibition by SNORD88C overexpression. Besides, we also observed the effects of SNORD88C and SCD1 on mTOR pathway. As expected, SCD1 abolished the effect of SNORD88C on mTOR pathway related protein, including pMTOR, pP70S6K, pULK1 (Fig. 5F), indicating SNORD88C might promote SCD1 protein expression, then accelerate MUFA synthesis, inhibit lipid peroxidation, affect mTOR pathway and finally inhibit autophagy.
Finally, we examined the direct link between SNORD88C/SCD1 axis and migration and invasion of lung cancer cells. As shown in Figs. 5G, H, S6G-J, SCD1 overexpression reversed the inhibitory effect on migration and invasion in A549 and H1299 cells after SNORD88C silencing, while SCD1 knockdown abolished the promotion of SNORD88C overexpression on lung cancer cell metastasis in SPC-A1 and H1299 cells. Likewise, SCD1 abolished the effect of SNORD88C on EMT pathway-related protein, including N-cadherin, Vimentin, and E-cadherin (Fig. S6K). Taken together, these results suggest that SCD1 was the downstream regulatory protein of SNORD88C and promoted the migration and invasion of NSCLC cells by inhibiting autophagy.
SNORD88C regulates the translation activity of SCD1 though guiding 2′-O-me of 28S rRNA
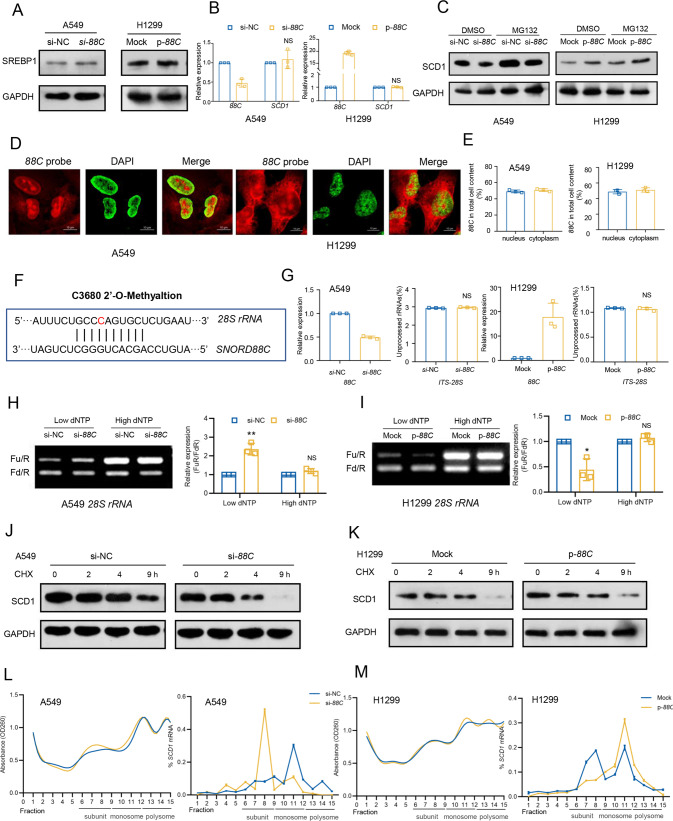
To explore the underlying mechanism involved in SNORD88C/SCD1 axis, we first detected the effect of SNORD88C on SREBP1, an important transcription factor that regulates the expression of SCD1 [16]. Unexpectedly, SNORD88C exerted no obvious effect on SREBP1 expression (Fig. 6A), indicating SNORD88C did not regulate transcription of SCD1, which was also confirmed by another observation that SNORD88C did not affect SCD1 mRNA level (Fig. 6B). Thereby, we speculated that SNORD88C regulated SCD1 expression at the post-transcriptional level. Next, the proteasome inhibitor MG132 was used to block proteasomal degradation. However, MG132 treatment clearly increased SCD1 protein levels, but failed to reverse the regulation of SNORD88C on SCD1 (Fig. 6C), suggesting a protein-degradation-independent manner involved.
Fig. 6. SNORD88C regulates the translation activity of SCD1 though guiding 2′-O-me of 28S rRNA.
A Western blot showed that SREBP1 expression was not affected by SNORD88C knockdown or overexpression. B q-PCR showed that SCD1 mRNA level was not affected by SNORD88C knockdown or overexpression. C Western blot showed MG132 treatment clearly increased SCD1 protein levels, but failed to reverse the regulation of SNORD88C knockdown or overexpression on SCD1. D, E Fluorescent in situ hybridization (FISH) using Cy3-labeled probes targeting SNORD88C and q-PCR showed SNORD88C was mainly located in the nucleolus and cytoplasm. Red represented SNORD88C, green (artifact) represented nucleus. Scale bar indicates 10 μm. F The snoPY database predicted the complementary sequence and 2′-O-me site of rRNA targeted by SNORD88C, and red represented the methylation site. G q-PCR assay to detect ITS-28S showed that SNORD88C knockdown (left) or overexpression (right) did not affect the processing of 28S rRNA. The value of the vertical coordinate represented the average of primer pairs d/c (unprocessed) over b/a (total) and primer pairs f/e (unprocessed) over b/a (total) for 28S rRNA, respectively. Primers were shown in Table S4. H, I The 2′-O-me activity of 28S rRNA at C3680 site detected by RTL-P assay was decreased after SNORD88C knockdown in A549, while increased after SNORD88C overexpression in H1299. J, K The translation activity of SCD1 was reduced by SNORD88C knockdown treated by the CHX in A549 cells, while increased by SNORD88C overexpression in H1299 cells. Two-tailed unpaired t test; ns, no significance; *P < 0.05, **P < 0.01. L, M Ribosomal components in cytoplasmic extracts of si-NC and si-88C A549 cells (L) or mock and p-88C H1299 cells (M) were fractionated through sucrose gradients, and the relative levels of SCD1 mRNA were analyzed by qRT-PCR in the gradient fractions.
Previous studies have demonstrated box C/D snoRNAs are essential players in the rRNA biogenesis due to their involvement in guidance of 2′-O-me in the nucleolus, in turn regulation of protein translation in the cytoplasm [6]. Firstly, we performed fluorescence in situ hybridization (FISH) to detect the cellular distribution using Cy3-labeled probes targeting SNORD88C. As shown in Figs. 6D, S7A, SNORD88C was mainly located in the nucleolus and cytoplasm. This result was further validated by q-PCR in cytoplasmic/nuclear fractionation (Figs. 6E, S7B), indicating the potential of SNORD88C to directly regulate rRNA biological properties.
Moreover, we predicted SNORD88C-targeted rRNA through the snoPY database (http://snoopy.med.miyazaki-u.ac.jp/) based on bioinformatics, and found only 28S rRNA contained a high complementary sequence to SNORD88C, and more important a potential site of c3680 for its-guided 2′-O-me (Fig. 6F). Then, primers that could distinguish the precursor (ITS-28S) and total 28S rRNA were used to quantify, nevertheless, no significant changes of mature 28S rRNA were observed neither in SNORD88C-knockdown A549 cells nor in SNORD88C-overexpression H1299 cells (Table S4, Fig. 6G). Next, RTL-P assay, an approach based on reverse transcription with limiting dNTP concentration (Table S5), was used to confirm SNORD88C-guided 2′-O-me of 28S rRNA. SNORD88C knockdown dramatically reduced the 2′-O-me of 28S rRNA at the C3680 site under the lower dNTPs concentrations but not under the higher dNTPs in A549 cells (Fig. 6H). Meanwhile, overexpression of SNORD88C elevated the 2′-O-me activity under the lower dNTPs concentrations (Fig. 6I). Previous studies had demonstrated 2′-O-me would lead to the enhancement of rRNA-induced translational accuracy, and then prolong protein half-life [6, 17–20]. Thereby, in vitro translation assay was performed via addition of cycloheximide (CHX), an inhibitor of protein translation to block overall protein synthesis. As a consequence, the half-life of SCD1 protein was shortened in SNORD88C-knockdown A549 cells (Fig. 6J) but extended in SNORD88C-overexpression H1299 cells (Fig. 6K) when treated with CHX. Finally, to figure out the possibility SNORD88C affected translation directly, polysome profiling was analyzed. Ribosomes fractions in the cell lysate were divided into ribosomal subunits (40S and 60S), monosomes (80S) and polysomes. As shown in Fig. 6L, M, SNORD88C silencing or overexpression did not affect the distribution profile of ribosomes fraction (left), indicating that SNORD88C did not influence global translation. Nevertheless, SNORD88C silencing significantly decreased SCD1 mRNA enrichment in the monosomes and polysomes fractions, altering its distribution from the heavier to the lighter ribosome fractions, whereas SNORD88C overexpression demonstrated the opposite effect (right), more directly proving that SNORD88C regulated the expression of SCD1 by affecting translation level.
Discussion
A myriad of studies emerged associating snoRNA dysregulation with cancer progression [21]. In present study, we identified the crucial role of SNORD88C in NSCLC, SNORD88C was up-regulated in tissue and plasma and served as a non-invasive diagnostic biomarker for NSCLC. Besides, through loss of function and functional acquisition analysis, we confirmed that SNORD88C promoted the proliferation and invasion, and metastasis of NSCLC in vivo and in vitro. More importantly, SNORD88C-mediate 2′-O-me of 28S rRNA was essential for the initiation and maintenance of cancer.
2′-O-me of rRNA is the most common chemical modification of SNORDs and the supplement to the mechanism in tumorigenesis such as in colorectal cancer, leukemia and liver cancer [8, 9, 22], but rarely in NSCLC. In current study, we demonstrated that SNORD88C regulated the translation of SCD1 activity though guiding 2′-O-me of 28S rRNA. To illuminate this issue, we first confirmed SNORD88C was capable to increase SCD1 expression but not at the transcriptional level, because SNORD88C had no impact on the expression of SCD1 mRNA and its upstream transcription factor SREBP1; Moreover, the proteasome inhibitor MG132 treatment failed to reverse the regulation of SNORD88C on SCD1, suggesting a protein-degradation independent manner involved. Direct evidence was obtained from RTL-P assay. Only 28S rRNA contained a high complementary sequence to SNORD88C with a potential site of C3680 for its-guided 2′-O-me, through which SNORD88C dramatically elevated the 2′-O-me activity of 28S rRNA under the lower dNTPs concentrations but not under the higher dNTPs concentration. Translation inhibition experiments also proved that the half-life of SCD1 protein was prolonged by SNORD88C after the addition of CHX. In addition, polysome profiling assay more fully confirmed that SNORD88C regulated the expression of SCD1 protein by altering the distribution of SCD1 mRNA in ribosomal fractions without affecting global translation. These results support that SNORD88C affect the translation fidelity of SCD1 through regulating 2′-O-me activity of 28S rRNA at C3680.
SCD1, a rate-limiting enzyme responsible for MUFA synthesis, catalyzes the desaturation of saturated fatty acids [23]. Indeed, we proved SNORD88C induced abnormal lipid peroxidation dependent on SCD1 expression, because the inhibitory effect of SNORD88C on lipid peroxidation could be successfully reversed by SCD1. In previous studies, many snoRNAs regulated lipid metabolism in a 2′-O-Me -independent manner. For example, U32A, U33, U34, and U35A participated in lipid metabolism at the post-transcriptional level in the cytoplasm by binding to target RNA [24]. Herein, we proved that SNORD88C regulated abnormal lipid metabolism dependent on it guided the 2′-O-me of 28S rRNA.
Accumulating evidence have proved the connection between SCD1 and the autophagic process based on it regulated lipid abnormalities [25]. Autophagy, a catabolic process that provides cells with tremendous metabolic plasticity and a major reactive survival mechanisms by protecting cells from stress factors such as nutritional deprivation [26], is regulated by various metabolic imbalances [27]. However, the controversial role of SCD1-mediated autophagy was observed in cancer. For example, SCD1 inhibition led to a mild autophagy reduction in cervical cancer HeLa cells [28], but an opposite trend was reported in the colon cancer cell line HCT-116 [29]. In current study, we demonstrated SNORD88C increased SCD1 translation, in turn, exerted inhibitory role in autophagy. This was because SCD1 maintained mTOR activity through modulation of MUFA availability, which prevented overactivation of autophagy, consistent with the previous study [30]. Nevertheless, autophagy is implicated in both metastasis-suppressive and -promoting biological activities in cancer [31]. For example, autophagy promoted liver cancer cell invasion by activating TGF-β/Smad3 signaling under starvation [32] but suppressed breast cancer metastasis by degrading NBR1 [33]. Our results confirmed that SNORD88C/SCD1 inhibited autophagy, then promoted lung cancer metastasis, consistent with the previous studies autophagy inhibition in multiple mammary cancer models accelerates the proliferation of disseminated tumor cells (DTCs) into overt macro-metastases [31]. One plausible explanation is that autophagy is required to maintain tumor cells in a dormant state and cellular homeostasis [34, 35], thus restricted the different phenotypic transitions of tumor cell; another is that autophagy degraded metastasis-related molecules [36] or specific tissue type [37] led to the inhibition of metastasis by autophagy. However, the more precise reasons need to be further studied to discuss.
Taken together, we found that SNORD88C was a new oncogenic snoRNA in NSCLC. We proved SNORD88C was up-regulated in tissue and plasma and served as a non-invasive diagnostic biomarker; then confirmed that it promoted proliferation and metastasis of NSCLC in vitro and in vivo. Importantly, SNORD88C guided 28S rRNA methylation, which significantly increased the translation activity of downstream target gene SCD1. As a rate-limiting enzyme responsible for MUFA synthesis, SCD1 inhibited autophagy to further promote metastasis, providing the novel insight into the regulation of SNORD88C in NSCLC.
Materials and methods
Patients and Healthy donors
A total of 989 NSCLC cases with available clinical information and 91 matched normal lung tissues were included in the LUAD (n = 513) and LUSC (n = 476) cohorts of The Cancer Genome Atlas (TCGA). The snoRNAs gene expression data set of the above samples was downloaded from the SNORic database (http://bioinfo.life.hust.edu.cn/SNORic). FFPE samples of NSCLC and paired para-cancerous tissue from total 48 NSCLC patients and plasma samples from a total of 178 healthy volunteers and 224 NSCLC patients were collected from Shandong Cancer Hospital between September 2018 and July 2019. All patients didn’t receive any anti-tumor treatment before samples collection, or suffer any other endocrine, immune, or metabolic diseases. The healthy donors did not present any disease. Detailed clinicopathological information was provided in Tables 1, 2, S1. The diagnosis of cancer was confirmed by two pathologists. Tumor stage was determined according to the 8th edition of the lung cancer TNM staging standards formulated by IASLC.
Table 1.
Characteristics of NSCLC patients for SNORD88C in FFPE specimens.
| Parameters | Samples, n | SNORD88C expression | |
|---|---|---|---|
| Δ Ct Median (interquartile range) | P-value | ||
| Age (years) | |||
| ≤62 | 24 | 5.378 (4.240–5.873) | 0.0609 |
| >62 | 24 | 4.695 (3.951–5.038) | |
| Gender | |||
| Male | 32 | 4.855 (3.994–5.561) | 0.5053 |
| Female | 16 | 4.885 (4.396–5.921) | |
| Smoking | |||
| NO | 18 | 5.065 (4.240–5.814) | 0.5316 |
| YES | 27 | 4.915 (3.945–5.580) | |
| Not Available | 3 | ||
| Drinking | |||
| N0 | 23 | 5.115 (4.210–5.975) | 0.3300 |
| YES | 21 | 4.915 (3.958–5.463) | |
| Not Available | 4 | ||
| Histology | |||
| AC | 24 | 5.065 (4.049–5.953) | 0.5362 |
| SCC | 24 | 4.775 (4.101–5.484) | |
| Tumor size | |||
| V > 6 cm3 | 22 | 5.080 (4.200–5.689) | 0.9955 |
| V ≤ 6 cm3 | 23 | 4.710 (3.970–5.760) | |
| Not available | 3 | ||
| T stage | |||
| T1 | 26 | 5.065 (4.150–5.848) | 0.3108 |
| T2 | 14 | 4.525 (2.880–5.401) | |
| T3 | 3 | 5.300 (4.915–5.505) | |
| T4 | 2 | 5.595 (5.045–6.145) | |
| TX | 3 | ||
| LN meta. | |||
| N0 | 32 | 5.080 (4.066–5.734) | 0.3334 |
| N1 | 4 | 5.265 (4.331–6.000) | |
| N2 | 4 | 3.803 (-0.7300–5.046) | |
| N3 | 4 | 5.015 (2.344–6.651) | |
| NX | 4 | ||
| Distant meta. | |||
| M0 | 44 | 5.030 (4.229–5.734) | |
| M1 | 1 | 1.560 | |
| MX | 3 | ||
| TNM stage | |||
| I | 25 | 5.115 (3.958–5.823) | |
| II | 9 | 4.915 (4.283–5.670) | |
| III | 9 | 5.045 (3.803–5.740) | |
| IV | 1 | 1.560 | |
| Not available | 4 | ||
SnoRNA-sequencing
The snoRNA-sequencing and data analysis were conducted by Aksomics (Shanghai, China). Total RNAs were extracted from 10 plasma samples (4 healthy volunteers and 6 NSCLC patients, including 3 early-stage and 3 advanced-stage patients). Agarose electrophoresis was used to check the integrality of total RNA samples, and then the samples were quantified on the NanoDrop ND-1000 instrument. 1~2ug total RNA of each sample was taken for SnoRNAseq library preparation. The library was denatured with 0.1 M NaOH to generate single-stranded DNA molecules, and amplified in situ using NovaSeq 6000 S4 Reagent Kit. Sequencing was carried out by running 150 cycles on Illumina NovaSeq 6000 according to the manufacturer’s instructions. Sequencing quality was examined by FastQC software and trimmed reads were aligned to the Ensemble using NovoAlign software (v2.07.11). The expression profiling and differential expression of snoRNAs were calculated based on normalized TPM [38]. Hierarchical clustering, scatter plots and classification analysis were performed with the differential snoRNAs in R or Perl environment for statistical computing and graphics. Differential snoRNAs with statistical significance between the two groups were identified through p-value≤ 0.05.
Cell culture
Human NSCLC cells A549, SPC-A1 and H1299 were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) or American Type Culture Collection (Manassas, VA, USA), and cultured in DMEM medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Gibco) and antibiotics (penicillin/streptomycin, 100 U/ml) at 37 °C, 5% CO2. Mycoplasma PCR testing of these cells was performed every month.
Transfection and siRNA interference
Human full-length SNORD88C cDNA (NR_003069.1) was synthesized by Genewiz (Suzhou, China) and cloned into the pCDH-CMV-MCS-EF1α-Puro vector (SBI, CD510B), named as p-SNORD88C; SCD1 cDNA and ATG5 cDNA were both synthesized by GenePharma (Shanghai, China) and respectively cloned into the pcDNA3.1(+) vector and p-ENTER vector, named as p-SCD1 and p-ATG5. siRNAs of SNORD88C, ATG5, and SCD1, as well as the negative control RNA (si-NC) were purchased from Genepharma (Shanghai, China), sequences of siRNAs were listed in Table S3. Transfection of expressive vectors and siRNA interference were performed using Lipofectamine 3000 according to the manufacturer’s protocols (Invitrogen).
For stable SNORD88C KD, lentiviruses expressing shRNA hairpins targeting human SNORD88C and firefly luciferase was designed and produced based on reporter vector CV344 by Genechem Company (Shanghai, China), named LV-sh-SNORD88C. A549 cells were transfected with 5 μl lentivirus with titer of 2 × 108 Tu/ml lentiviral plasmid containing or not containing the target gene. The medium containing 5 μg/ml puromycin was used for screening after co-culture for 48 h. The medium was replaced once 1-2 d to continue pressurized screening for a total of 10 d.
RNA extraction, reverse transcription and q-PCR
Total RNAs of cells and plasma were isolated by adding Trizol and Trizol LS, respectively (Thermo Fisher Scientific, MA, USA) according to manufacturer’s instructions. In some experiments, nucleus, and cytoplasm needed to be separated first using Nuclear & Cytoplasmic RNA purification kit (NORGEN, Ontario, Canada). Total RNAs of FFPE samples were extracted using miRNAprep Pure FFPE kit (Tiangen Biotech, Beijing, China).
The extracted RNA was reverse transcribed to cDNA using Mir-X™ miRNA First-Strand Synthesis Kit and PrimeScript™ II 1st Strand cDNA Synthesis Kit (TaKaRa Bio, Nojihigashi, Kusatsu, Japan). qPCR was performed using TB-Green Premix Ex Taq II Reagent (TaKaRa Bio) and the primers for genes were presented in Table S2. Transfection efficiency was assessed using the 2−ΔΔCt method and relative quantification was analyzed using ΔCt (Δ Ct = CtsnoRNA-CtU6) method. U6 was used as the endogenous control of SNORD88C, while actin as the control of SCD1, ATG5 and C19orf48.
In vitro proliferation, colony formation, wound-healing, migration and invasion assays
For cell proliferation assay, twenty-four hours following transfection, 1000–3000 transfected NSCLC cells were incubated in 96-well-plate for indicated time points and OD value was measured at 450 nm using Cell Counting Kit-8 every 24 h according to the manufacturer’s instructions. Cell viability was calculated according to the formula: experimental OD value/control OD value × 100%
For colony formation assay, 100–200 transfected NSCLC cells were plated on a 6-well plate for 2 weeks. Cell colonies were fixed with 4% fixative solution (Solarbio, Beijing, China) and stained with gentian violet solution at room temperature for 30 min. colonies that consisted of >50 cells were counted and calculated. The colony formation efficiency was calculated with the following formula: Survival Fraction = Clones/Cell numbers × 100%.
For wound-healing assay, the transfected NSCLC cells were grown to a single layer covering the ground in six-well plate. A sterile 200 μl pipette tip was used to scrape the cell surface to create artificial wounds. The wound healing area was recorded under the microscope (Olympus, Japan) at three time points and analyzed using Fiji ImageJ software.
For migration assay, 5 × 104 transfected NSCLC cells were seeded in the upper chamber of transwell inserts (Corning, NY, USA) containing a polycarbonate membrane with 8.0 μm pores under serum-free conditions. The lower chamber was filled with 700 μl normal medium. After incubation for indicated time, cells on the upper surface of the membrane were completely removed by wiping with a cotton swab and fixed with 4% paraformaldehyde and stained with gentian violet for 30 min, then counted under the microscope (Olympus, Japan). For invasion assay, the transwell inserts were coated with Matrigel matrix (BD Science, USA).
Western blots
NSCLC cells were lysed in Cell Lysis Buffer (Beyotime, Beijing, China) supplemented with 0.5 mM PMSF (Beyotime), and the total cellular protein concentration was determined with a BCA Protein Assay Kit (Thermofisher). 50 μg protein was separated on SDS-PAGE and transferred onto PVDF membranes (Millipore, Billerica, MA, USA). Membranes were then blocked in TBST containing 5% evaporated skimmed milk (Bio-rad, USA) for 2–4 h, and then incubated overnight at 4 °C with the following primary antibodies: antibodies against human E-cadherin (14472), N-cadherin (13116), Vimentin (5741), LC3B (3868), SQSTM1 (88588), pAKT (4060), pAMPK (50081), pMTOR (5536), pP70S6K (9234). pULK1 (14202), Snail (3879), SCD1 (2794) (all 1:1000; Cell Signaling Technology, CST, Massachusetts, USA), GAPDH (10494–1-AP) (1:1000, Proteintech Group), SREBP1 (sc-13551) (1:100, Santa Cruz Biotechnology), followed by incubation with horseradish peroxidase coupled secondary anti-mouse (7076) or anti-rabbit antibodies (7074) (1:2000; CST) for 1 h at room temperature. The protein bands were visualized using ECL blotting detection reagents (Bio-Rad, USA), and then developed and fixed onto x-ray films. GAPDH was served as a loading control. In some experiments, 50 μg/mL CHX was added to the culture medium, followed by incubation for 0 h, 2 h, 4 h, or 9 h.
A549 tumor xenografts model and pulmonary metastasis model
For tumor xenografts model, sixteen 6-week-old male BALB/c-nude mice purchased from Beijing HFK Bioscience Co. Ltd. (Beijing, China) were randomly divided into two groups, a total of 5 × 106 A549 cells with LV-sh-NC or LV-sh-SNORD88C were respectively injected subcutaneously into the right flank region of each mouse. After visible tumors had developed, tumor volume was measured and calculated as (length × width2)/2 every 2 days. Five weeks after injection, the mice were sacrificed, and the tumor nodules were harvested and weighed.
For pulmonary metastasis model, ten 6-week-old male BALB/c-nude mice purchased from Beijing HFK Bioscience Co. Ltd. (Beijing, China) were randomly divided into two groups and a total of 1 × 106 A549 cells with stable firefly luciferase expression and silenced SNORD88C (LV-sh-SNORD88C), as well as the negative control (LV-sh-NC) were injected to the tail vein. Bioluminescent NSCLC metastases were monitored weekly via the IVIS Spectrum In Vivo Imaging System (PerkinElmer, USA). One month after injection, the mice lungs were harvested, fixed, weighed and stained. These mice were purchased from HFK Bioscience (Beijing, China). The Institutional Animal Care and Use Committee of Shandong Cancer Hospital and Institute approved these experiment procedures.
Immunohistochemistry
The paraffin-embedded tissues were cut into 4-μm sections and then analyzed by immunohistochemistry (IHC) using antibodies against Ki67 and CASPAS 3. The staining intensity and corresponding percentage were evaluated independently by two pathologists to confirm the reproducibility of the results. The intensity of staining was scored on a 0–2 scale, with no staining = 0, weak staining = 1, and strong staining = 2. The staining score was calculated according to the formula: IHC score = P1 × 1 + P2 × 2 + P3 × 3 (P: percentage).
Immunofluorescence (IF) staining and fluorescent in situ hybridization (FISH)
For IF staining, NSCLC cells were fixed with 4% paraformaldehyde after adherent growth on the climbing slice and permeabilized with 0.1% Triton X-100 for 15 min. After incubation with primary antibody against human anti-LC3B (CST) for 1 h, cells were incubated with Alexa 488-conjugated (1:1000) (Abcam) secondary antibodies for 1 h. Nucleus was stained with DAPI for 3 min (Beyotime). Images were acquired on a confocal laser microscopy (LSM800, Carle Zeiss, Germany) For quantification of the number of autophagosomes (diameters 0.3–1.0 μM), at least three cells were randomly chosen, all eligible puncta were recorded and analyzed using Fiji ImageJ software.
For FISH, SNORD88C-specific Cy3 fluorescence-labeled probe (5′-3′: TGTGTCCTCAGGGGTGATCAGAGCC) and fluorescence in situ hybridization kit (Genepharma, Shanghai, China) were used in FISH experiment. Briefly, the cells were inoculated on small chamber slides, fixed with 4% paraformaldehyde, and then exposed to 0.5% TritonX-100. After denaturation at 73 °C for 5 min, the probe was added to the cell culture medium and hybridized and incubated overnight After full washing, the nucleus was stained with DAPI. The image was captured on confocal laser microscopy.
Transmission electron microscopy (TEM)
TEM samples preparation and observation were performed by Weiya biotech Co., Ltd (Jinan, PR China). The NSCLC cells were fixed in 4% glutaraldehyde solution at 4 C for 4 h and washed with 0.1 M phosphoric acid buffer (PH7.4) for 3 times, each time 15 min. After fixation in 1% osmium tetroxide for 2 h, the specimens were washed with 0.1 m phosphoric acid buffer again. After dehydrated through a graded series of ethanol, the samples were embedded in Epon (Sigma, 45347). Ultrathin sections were stained with 2% uranyl acetate and lead citrate, and observed using a transmission electron microscope (HT7700, Hitachi, Japan).
Adenoviral infection
Recombinant adenoviral vector carrying the human mRFP-GFP-LC3 gene was purchased from HanBio (Shanghai, China). Cells were plated in 12-well plates at a density of 1 × 104 cells per well and infected at an MOI of 2 with GFP-mRFP-LC3 gene for 24 h. After washing with PBS twice, cells were transfected with si-NC or si-88C or treated with rapamycin or Baf.A1 for another 24 h respectively.
LC-MS/MS-based proteomics and GC-MS-based fatty acids metabolomics
For LC-MS/MS-based proteomics, the cultured H1299 cells were divided into two groups and transfected with si-SNORD88C and si-NC respectively. The proteins of the two groups were extracted and identified by TMT labeling, high-performance liquid chromatography (HPLC), and quantitative proteomics based on mass spectrometry (MS). All the identified proteins were analyzed systematically by bioinformatics, and all differential proteins were analyzed by functional classification, functional enrichment, and cluster analysis based on functional enrichment. Extraction, detection, and analysis of proteins are performed by Jingjie PTM Biolab Co. Ltd (Hangzhou, PR China).
For GC-MS-based fatty acids metabolomics, H1299 cells were divided into two groups transfected with si-SNORD88C or si-NC, and 6 independent samples were needed for the corresponding biological repeats of each group. All samples were quantified by BCA and then the abundance of free fatty acids was detected on the GC/MS platform. Detection and analysis are performed by Shanghai Biotree biotech Co. Ltd. (Shanghai, PR China). The student’s t-test (p < 0.05) was used to assess the significance of difference in abundance of free fatty acids between the two groups.
Measurement of lipid peroxidation
After, cells were stained with 5 μM C11-bodipy 581/591 lipid peroxidation fluorescent probe (ABclonal, RM02821) for 30 min, then digested with trypsin, re-suspended in PBS containing 5% FBS and analyzed by flow cytometry. The proportion of lipid peroxidation cells was quantified according to the arithmetic mean value ± SEM of histogram fluorescence signal.
RTL-P assay for rRNA 2′-O-methylation
2′-O-me of 28S rRNA at C3680 site was measured as previously reported with minor modification [39]. The mixture containing specific RT primers, total RNA, and a low (10 μM) or high (1 mM) concentration of dNTPs was heated at 65 °C for 5 min and then was placed on ice, the specific primers were shown in Table S5. Next, 5 × PrimeScript II Buffer, RNase Inhibitor and PrimeScript II RTase were mixed into the reaction solution of the first step with an initial annealing step at 42 °C for 1 h and then mixture was heated at 95 °C for 5 min. The PCR reaction was determined using 2 × Taq Plus Master Mix II (Dye Plus) (Vazyme, Nanjing, China), and the agarose gel electrophoresis was carried out to detect rRNA methylation.
Polysome profiling
5 × 107 NSCLC cells were exposed to cycloheximide (200 µg/ml) for 5 min, and then were sufficiently lysed in 1000 µl of cytoplasmic lysis buffer containing polysome buffer 878 μl (1000 μl = 3 M NaCl 50 μl, 1 M MgCl2 5 μl, 1 M Tris HCL Ph7.5 20 μl, RNase free H2O 925 μl), 10% Triton X-100 100 μl, 100 mM DTT 10 μl, 1U/μl DNase I 10 μl, 50 mg/ml cycloheximide 2 μl. After centrifugation at 2000 g at 4 °C for 15 minutes, the supernatant was observed and supplemented with the lysis buffer to make the RNA concentration in the supernatant of different samples consistent. RNA concentration was detected by Nano Drop one (Thermo Fisher Scientific, MA, USA).
For fractionation, the same volume supernatants were loaded into 10%-45% sucrose gradients and separated by ultracentrifugation with a SW41Ti rotor (Beckman Coulter, Brea, CA, USA) at 4 °C and 230,000 g for 3 hours. The centrifuged solution was equally divided into 15 equal fractions from top to bottom and then the OD 260 value of each fraction was measured. Subsequently, total RNA was extracted from each fraction, then subjected to RT-qPCR for SCD1 mRNA analysis. Relative distribution of mRNA in each fraction was normalized with the sum of all fractions.
Statistical analysis
All the analyses were completed by SPSS 21.0 software (IBM, Ehningen, Germany) and GraphPad Prism version 8.0 (San Diego, CA, USA), with P < 0.05 being considered statistically significant. The normality of the distribution was checked using the Kolmogorov–Smirnov test. If the data followed normal analysis, unpaired t-test would be used; if not, Mann-Whitney test would be used. Chi-square test was used for analyzing categorical variables. Multi-group analysis was tested by one-way analysis of variance (ANOVA) or Kruskal-Wallis test. In paired data, the normally distributed numeric variables were evaluated by paired t-test, whereas non-normally distributed variables were analyzed by Wilcoxon rank-test. Receiving operating characteristic (ROC) curve was used to evaluate diagnostic efficiency. The data was presented as the median with interquartile range.
Supplementary information
revised supplementary tables(SNORD88C)(RR)
Original western blots Fig.6J,K(revised)
Acknowledgements
We thank Hualei Yu for technical assistance of polysome profiling experiment and Xiujie Cui for the animal care.
Author contributions
SX and SX designed and conceived the research content. Wang Kangyu performed sample collection, experiments, data analysis and wrote the manuscript. SX guided the experiments and reviewed the manuscript. WS carried out the Western blot assay. ZY extracted RNA of FFPE samples. SX, XL, and SX contributed reagents materials, instruments, analytic tools, and grant support. The author(s) read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81972014), the Shandong Provincial Natural Science Foundation (ZR2019MH004 and ZR2019LZL016) and Shandong Provincial Key Research and Development Program (Major Science & Technology Innovation Project) (2021SFGC0501).
Data availability
All relevant data are available from the corresponding author upon reasonable request. In addtion, raw data from snoRNA sequencing and protein mass spectrometry were uploaded to the GAS-Human (HRA003325) and OMIX (OMIX002173) databases of CNCB-NGDC (China National Center for Bioinformation-National Genomics Data Center).
Ethics statement
The studies involving human participants (201806004) and animals (201911025) were reviewed and approved by Ethics Committee of Shandong Cancer Hospital and Institute.
Competing interests
The authors declare no competing interests.
Footnotes
Edited by E. Baehrecke
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xingguo Song, Email: xgsong@sdfmu.edu.cn.
Xianrang Song, Email: xrsong@sdfmu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41418-022-01087-9.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–54.. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 3.Mannoor K, Liao J, Jiang F. Small nucleolar RNAs in cancer. Biochim Biophys Acta. 2012;1826:121–8. doi: 10.1016/j.bbcan.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang J, Wen J, Huang Z, Chen XP, Zhang BX, Chu L. Small nucleolar RNAs: Insight into their function in cancer. Front Oncol. 2019;9:587. doi: 10.3389/fonc.2019.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams GT, Farzaneh F. Are snoRNAs and snoRNA host genes new players in cancer? Nat Rev Cancer. 2012;12:84–8. doi: 10.1038/nrc3195. [DOI] [PubMed] [Google Scholar]
- 6.Erales J, Marchand V, Panthu B, Gillot S, Belin S, Ghayad SE, et al. Evidence for rRNA 2′-O-methylation plasticity: Control of intrinsic translational capabilities of human ribosomes. Proc Natl Acad Sci USA. 2017;114:12934–9. doi: 10.1073/pnas.1707674114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pelletier J, Thomas G, Volarevic S. Ribosome biogenesis in cancer: new players and therapeutic avenues. Nat Rev Cancer. 2018;18:51–63. doi: 10.1038/nrc.2017.104. [DOI] [PubMed] [Google Scholar]
- 8.Wu H, Qin W, Lu S, Wang X, Zhang J, Sun T, et al. Long noncoding RNA ZFAS1 promoting small nucleolar RNA-mediated 2′-O-methylation via NOP58 recruitment in colorectal cancer. Mol Cancer. 2020;19:95. doi: 10.1186/s12943-020-01201-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z, Pang Y, Jia Y, Qin Q, Wang R, Li W, et al. SNORA23 inhibits HCC tumorigenesis by impairing the 2′-O-ribose methylation level of 28S rRNA. Cancer Biol Med. 2021;19:104–19. [DOI] [PMC free article] [PubMed]
- 10.Okugawa Y, Toiyama Y, Toden S, Mitoma H, Nagasaka T, Tanaka K, et al. Clinical significance of SNORA42 as an oncogene and a prognostic biomarker in colorectal cancer. Gut. 2017;66:107–17.. doi: 10.1136/gutjnl-2015-309359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian B, Liu J, Zhang N, Song Y, Xu Y, Xie M, et al. Oncogenic SNORD12B activates the AKT-mTOR-4EBP1 signaling in esophageal squamous cell carcinoma via nucleus partitioning of PP-1alpha. Oncogene. 2021;40:3734–47.. doi: 10.1038/s41388-021-01809-2. [DOI] [PubMed] [Google Scholar]
- 12.Liao J, Yu L, Mei Y, Guarnera M, Shen J, Li R, et al. Small nucleolar RNA signatures as biomarkers for non-small-cell lung cancer. Mol Cancer. 2010;9:198. doi: 10.1186/1476-4598-9-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y, Yan Y, Ma R, Lv X, Zhang L, Wang J, et al. Expression signature of six-snoRNA serves as novel non-invasive biomarker for diagnosis and prognosis prediction of renal clear cell carcinoma. J Cell Mol Med. 2020;24:2215–28.. doi: 10.1111/jcmm.14886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shang X, Song X, Wang K, Yu M, Ding S, Dong X, et al. SNORD63 and SNORD96A as the non-invasive diagnostic biomarkers for clear cell renal cell carcinoma. Cancer Cell Int. 2021;21:56. doi: 10.1186/s12935-020-01744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klionsky DJ, Abdel-Aziz AK, Abdelfatah S, Abdellatif M, Abdoli A, Abel S, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)(1) Autophagy. 2021;17:1–382. doi: 10.1080/15548627.2020.1797280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi J, Zhu J, Wu J, Thompson CB, Jiang X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci USA. 2020;117:31189–97.. doi: 10.1073/pnas.2017152117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Miguel VE, Lujan C, Espie-Caullet T, Martinez-Martinez D, Moore S, Backes C, et al. Increased fidelity of protein synthesis extends lifespan. Cell Metab. 2021;33:2288–300.e12. doi: 10.1016/j.cmet.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrade JM, Dos Santos RF, Chelysheva I, Ignatova Z, Arraiano CM. The RNA-binding protein Hfq is important for ribosome biogenesis and affects translation fidelity. EMBO J. 2018;37:e97631. [DOI] [PMC free article] [PubMed]
- 19.Bustelo XR, Dosil M. Ribosome biogenesis and cancer: basic and translational challenges. Curr Opin Genet Dev. 2018;48:22–9. doi: 10.1016/j.gde.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Chaillou T. Ribosome specialization and its potential role in the control of protein translation and skeletal muscle size. J Appl Physiol (1985) 2019;127:599–607. doi: 10.1152/japplphysiol.00946.2018. [DOI] [PubMed] [Google Scholar]
- 21.Thorenoor N, Slaby O. Small nucleolar RNAs functioning and potential roles in cancer. Tumour Biol. 2015;36:41–53. doi: 10.1007/s13277-014-2818-8. [DOI] [PubMed] [Google Scholar]
- 22.Zhou F, Liu Y, Rohde C, Pauli C, Gerloff D, Kohn M, et al. AML1-ETO requires enhanced C/D box snoRNA/RNP formation to induce self-renewal and leukaemia. Nat Cell Biol. 2017;19:844–55.. doi: 10.1038/ncb3563. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Klein MG, Zou H, Lane W, Snell G, Levin I, et al. Crystal structure of human stearoyl-coenzyme A desaturase in complex with substrate. Nat Struct Mol Biol. 2015;22:581–5. doi: 10.1038/nsmb.3049. [DOI] [PubMed] [Google Scholar]
- 24.Caputa G, Schaffer JE. RNA regulation of lipotoxicity and metabolic stress. Diabetes. 2016;65:1816–23. doi: 10.2337/db16-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ascenzi F, De Vitis C, Maugeri-Sacca M, Napoli C, Ciliberto G, Mancini R. SCD1, autophagy and cancer: implications for therapy. J Exp Clin Cancer Res. 2021;40:265. doi: 10.1186/s13046-021-02067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimmelman AC, White E. Autophagy and tumor metabolism. Cell Metab. 2017;25:1037–43.. doi: 10.1016/j.cmet.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15:713–20. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogasawara Y, Itakura E, Kono N, Mizushima N, Arai H, Nara A, et al. Stearoyl-CoA desaturase 1 activity is required for autophagosome formation. J Biol Chem. 2014;289:23938–50. doi: 10.1074/jbc.M114.591065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ono A, Sano O, Kazetani KI, Muraki T, Imamura K, Sumi H, et al. Feedback activation of AMPK-mediated autophagy acceleration is a key resistance mechanism against SCD1 inhibitor-induced cell growth inhibition. PLoS One. 2017;12:e0181243. doi: 10.1371/journal.pone.0181243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou X, Zhu X, Li C, Li Y, Ye Z, Shapiro VS, et al. Stearoyl-CoA desaturase-mediated monounsaturated fatty acid availability supports humoral immunity. Cell Rep. 2021;34:108601. doi: 10.1016/j.celrep.2020.108601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen HT, Liu H, Mao MJ, Tan Y, Mo XQ, Meng XJ, et al. Crosstalk between autophagy and epithelial-mesenchymal transition and its application in cancer therapy. Mol Cancer. 2019;18:101. doi: 10.1186/s12943-019-1030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Yang B, Zhou Q, Wu Y, Shang D, Guo Y, et al. Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial-mesenchymal transition. Carcinogenesis. 2013;34:1343–51. doi: 10.1093/carcin/bgt063. [DOI] [PubMed] [Google Scholar]
- 33.Marsh T, Debnath J. Autophagy suppresses breast cancer metastasis by degrading NBR1. Autophagy. 2020;16:1164–5. doi: 10.1080/15548627.2020.1753001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferraresi A, Girone C, Esposito A, Vidoni C, Vallino L, Secomandi E, et al. How autophagy shapes the tumor microenvironment in ovarian cancer. Front Oncol. 2020;10:599915. doi: 10.3389/fonc.2020.599915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Prat L, Munoz-Canoves P, Martinez-Vicente M. Monitoring autophagy in muscle stem cells. Methods Mol Biol. 2017;1556:255–80.. doi: 10.1007/978-1-4939-6771-1_14. [DOI] [PubMed] [Google Scholar]
- 36.Lv Q, Wang W, Xue J, Hua F, Mu R, Lin H, et al. DEDD interacts with PI3KC3 to activate autophagy and attenuate epithelial-mesenchymal transition in human breast cancer. Cancer Res. 2012;72:3238–50. doi: 10.1158/0008-5472.CAN-11-3832. [DOI] [PubMed] [Google Scholar]
- 37.Cheong H, Lu C, Lindsten T, Thompson CB. Therapeutic targets in cancer cell metabolism and autophagy. Nat Biotechnol. 2012;30:671–8. doi: 10.1038/nbt.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bullard JH, Purdom E, Hansen KD, Dudoit S. Evaluation of statistical methods for normalization and differential expression in mRNA-Seq experiments. BMC Bioinforma. 2010;11:94. doi: 10.1186/1471-2105-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong ZW, Shao P, Diao LT, Zhou H, Yu CH, Qu LH. RTL-P: a sensitive approach for detecting sites of 2′-O-methylation in RNA molecules. Nucleic Acids Res. 2012;40:e157. doi: 10.1093/nar/gks698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
revised supplementary tables(SNORD88C)(RR)
Original western blots Fig.6J,K(revised)
Data Availability Statement
All relevant data are available from the corresponding author upon reasonable request. In addtion, raw data from snoRNA sequencing and protein mass spectrometry were uploaded to the GAS-Human (HRA003325) and OMIX (OMIX002173) databases of CNCB-NGDC (China National Center for Bioinformation-National Genomics Data Center).