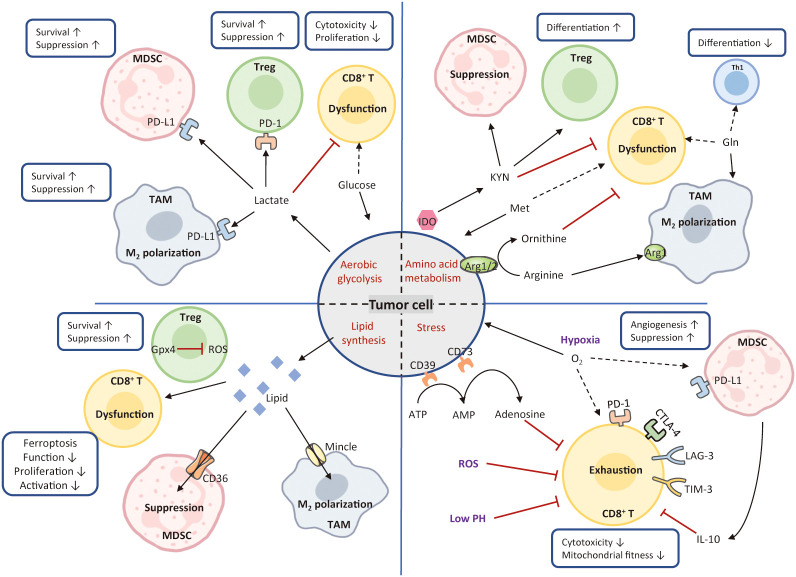
Figure 4.
Mechanisms of metabolism-mediated immune escape. In the TME, hypermetabolic tumor cells interfere with immune cell function by depriving nutrients and produce various types of metabolic stress. Tumor cells utilize large amounts of glucose and amino acids to fuel their glycolysis and amino acid metabolism. These activities greatly limit nutrient availability to T cells, leading to the formation of immunosuppressive TME. Tumor cells also release excessive lipids into the TME, resulting in the enhanced lipid metabolism, high oxidative stress, and T-cell dysfunction. Conversely, Treg cells express high levels of glutathione peroxidase 4, avoiding ROS accumulation and the induction of ferroptosis. Cancer metabolism produces various metabolic stimuli, including hypoxia, low PH, and ROS, all of which impede CD8+ cytotoxicity and fitness. The solid black arrows present that the majority of nutrients are consumed by the cells, whereas the dashed black arrows indicate a paucity of molecule available to the cells. The red arrows represent inhibited metabolic pathways. MDSC, myeloid-derived suppressor cell; Treg, regulatory T; TAM, tumor-associated macrophage; Th1, T helper 1; IDO, indoleamine 2, 3-dioxygenase; Arg1, arginase; ROS, reactive oxygen species; KYN, kynurenine; Met, methionine; Gln, glutamine; Gpx4, glutathione peroxidase 4.