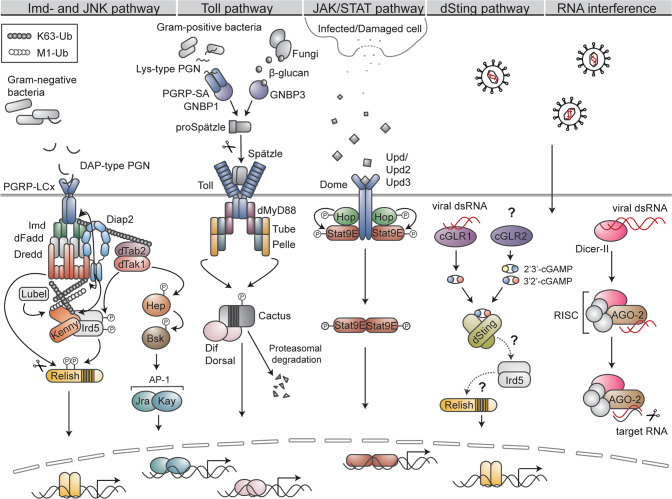
Fig. 2. Signalling pathways regulating Drosophila innate immunity.
The Imd pathway is initiated by DAP-type PGN derived from Gram-negative bacteria binding to the PGRP-LC receptor. The adaptor proteins Imd and dFadd, the caspase Dredd and the E3 ligase Diap2 are recruited to the receptor, whereafter Diap2 ubiquitinates Imd, Dredd and Kenny with K63-linked ubiquitin chains. The chains on Imd are thought to recruit the dTak1/dTab2 complex, whereas the chains on Dredd are needed for caspase activity and Relish cleavage. In addition to K63-linked chains, Kenny is also modified by M1-linked ubiquitin chains synthesised by Lubel. The dTak1/dTab2 complex is upstream of the IKK complex, consisting of Kenny and Ird5, which activates Relish by Ird5-mediated phosphorylation. The pathway culminates in translocation of Relish to the nucleus and target gene expression. In addition to driving Imd signalling, dTak1 functions as one of the apical-most kinases in the JNK pathway. Activated dTak1 phosphorylates Hep, that in turn phosphorylates Bsk. Bsk activates the transcription factor complex AP-1, consisting of Kay and Jra, which drives target gene expression after its nuclear translocation. The Toll pathway is induced when Lys-type PGN, originating from the cell wall of Gram-positive bacteria, is recognised by the PGRP-SA/GNBP1 receptor complex or when β-glucan, derived from fungi, is sensed by GNBP3. The activated receptors induce a serine-cascade culminating in the maturation of the ligand Spätzle from proSpätzle, whereafter Spätzle is recognized by the Toll receptor. Receptor activation leads to the recruitment of dMyD88, Tube and Pelle and subsequent Pelle-mediated phosphorylation of the IκB protein Cactus, targeting the protein for proteasomal degradation. Freed transcription factors Dif and Dorsal translocate to the nucleus and drive target gene expression. JAK/STAT signalling is activated by the cytokines Upd, Upd2 and Upd3, secreted by infected or damaged neighbouring cells. The Upds bind the receptor Dome, leading to phosphorylation of receptor-associated Hop. Activated Hop phosphorylates the transcription factor Stat9E, hence inducing its dimerisation and nuclear translocation. The dSting pathway is activated by viral dsRNA, sensed by the receptor cGLR1. The activated receptor produces 3’2’-cGAMP, a secondary messenger activating dSting. Signalling downstream of dSting remains largely elusive, however Ird5 and Relish seem to be needed for dSting target gene expression. In addition to cGLR1, cGLR2 is known to produce both 2’3’-cGAMP and 3’2’-cGAMP, however, its upstream trigger remains unknown. Viral dsRNA is recognised and cut into smaller fragments by Dicer-II. The fragments are loaded into AGO-2-containing RNA-induced silencing complex (RISC) wherein one of the viral RNA strands is degraded. The activated RISC complex then recognises and degrades RNA containing complementary sequences to the original viral RNA.